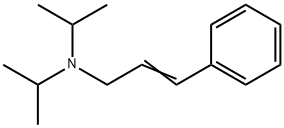
N,N-Bisisopropyl-3-phenyl-2-propenaMine synthesis
- Product Name:N,N-Bisisopropyl-3-phenyl-2-propenaMine
- CAS Number:87462-12-2
- Molecular formula:C15H23N
- Molecular Weight:217.35
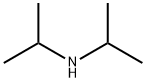
108-18-9
385 suppliers
$10.00/1g
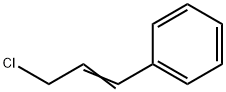
2687-12-9
167 suppliers
$5.00/5g

87462-12-2
12 suppliers
$130.00/1g
Yield:87462-12-2 93%
Reaction Conditions:
with potassium carbonate;potassium iodide in methanol;toluene; for 20 h;Reflux;
Steps:
1
A mixture of cinnamyl chloride (905 ml, 6.5 mol), diisopropylamine (1.37 1, 9.75 mol), potassium carbonate (0.90 kg, 6.5 mol), potassium iodide (54 g, 0.325 mol), toluene (2.1 l) and methanol (0.50 1) is stirred at reflux temperature for 20 hours. The mixture is cooled to 25 °C and water (5.2 1) is added. Phases are separated and the organic phase is extracted with brine. Organic phase is concentrated under reduced pressure (50 °C) and then water (10.4 1) and toluene (2.6 l) are added and the pH is adjusted to 2 by addition of concentrated hydrochloric acid (-500 ml). The resulting mixture is stirred for 15 minutes and the phases are separated. The aqueous phase is re-extracted twice with toluene (2 x 2.6 l). Then the pH of the aqueous phase is adjusted to 12 by addition of 8 M aqueous sodium hydroxide solution (750 ml). To the resulting white suspension is added heptane (5.2 l) and the mixture is stirred for 15 minutes. Phases are separated and aqueous phase is re-extracted twice with heptane (2 x 2.6 l). Combined organic phases are dried over Na2SO4 and concentrated to give 1.32 kg (93 % yield) of DIPCA.
References:
EP2364966,2011,A1 Location in patent:Page/Page column 17-18
673-32-5
136 suppliers
$11.00/1g

108-18-9
385 suppliers
$10.00/1g

87462-12-2
12 suppliers
$130.00/1g
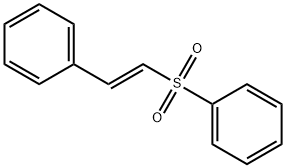
16212-06-9
58 suppliers
$25.00/1g
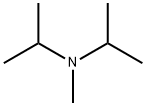
10342-97-9
64 suppliers
$41.00/5g

87462-12-2
12 suppliers
$130.00/1g
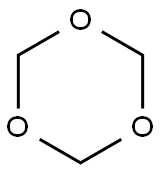
110-88-3
263 suppliers
$13.00/100g
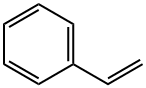
100-42-5
669 suppliers
$23.00/25mL

108-18-9
385 suppliers
$10.00/1g
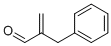
30457-88-6
7 suppliers
inquiry

87462-12-2
12 suppliers
$130.00/1g
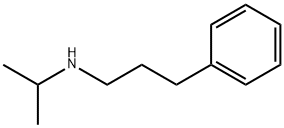
87462-11-1
8 suppliers
$502.25/5MG
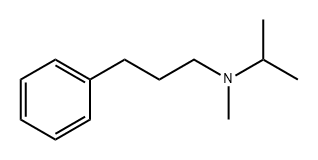
105864-96-8
0 suppliers
inquiry