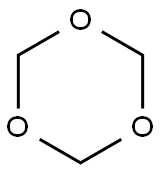
1,3,5-trioxane synthesis
- Product Name:1,3,5-trioxane
- CAS Number:110-88-3
- Molecular formula:C3H6O3
- Molecular Weight:90.08

64-17-5
825 suppliers
$10.00/50g

110-88-3
263 suppliers
$13.00/100g

75-07-0
429 suppliers
$14.00/5mL
Yield:-
Reaction Conditions:
with silicon carbide at 150; under 750.075 Torr; for 3 h;
Steps:
General procedure: All the catalysts were tested with the aim to determine their activity and selectivity in the oxidation (oxidative dehydrogenation, ODH) of ethanol. A trickle bed reactor (stainless steel 316) with a length of 1000 mm was used. The reaction was carried out using 2.2 g of catalyst. The catalyst was prepared in a form of pellets with a diameter of 0.5 mm. The catalyst bed had a length of 301 mm and was located in the central part of the reactor. The catalyst and silicon carbide (0.5 mm particles) were mixed thoroughly(20 ml SiC + 2.2 g of catalyst) and loaded into the reactor. Finally, the remaining free volume of the reactor was filled with silicon carbide. Air flow, used for the catalytic reaction, was fed directly (activation1 h at 400 °C; 5 °C/min from room temp.) or mixed with feed stock before entering the reactor. All catalysts were tested at 150, 200,225 and 250 C using 5 NL/h air flow, 1 bar of pressure and 5 g/h of ethanol. V/TiO2 catalyst was also tested using larger times of reaction. The products were collected in two collectors, the first one cooled by water to room temperature and the second one cooled to 0 °C. Each two collected liquid samples were mixed to one sample and analysed using a GC-FID “Agilent 7890A” and GC-OFID “Agilent-Wasson-ECE Instrumentation”. Gaseous products were analysed by the method “Refinery Gas Analysis” RGA (Agilent Technologies)with a GC 7890A Agilent (USA). The products were identified by using standard reference compounds along with GC-MS analyses using Thermo Scientific ITQ 1100 unit.
References:
Hidalgo;Ti?ler;Kubi?ka;Raabova;Bulanek [Journal of Molecular Catalysis A: Chemical,2016,vol. 420,p. 178 - 189]

50-00-0
896 suppliers
$10.00/25g

110-88-3
263 suppliers
$13.00/100g

64-17-5
825 suppliers
$10.00/50g

110-88-3
263 suppliers
$13.00/100g
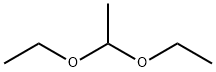
105-57-7
317 suppliers
$19.00/25mL

64-17-5
825 suppliers
$10.00/50g

110-88-3
263 suppliers
$13.00/100g

105-57-7
317 suppliers
$19.00/25mL

75-07-0
429 suppliers
$14.00/5mL

64-17-5
825 suppliers
$10.00/50g

110-88-3
263 suppliers
$13.00/100g

75-07-0
429 suppliers
$14.00/5mL

64-19-7
1628 suppliers
$10.00/25ML