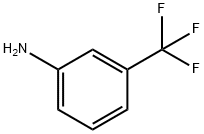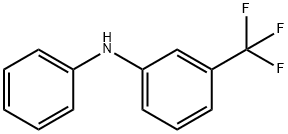
N-phenyl-3-(trifluoromethyl)aniline synthesis
- Product Name:N-phenyl-3-(trifluoromethyl)aniline
- CAS Number:101-23-5
- Molecular formula:C13H10F3N
- Molecular Weight:237.22

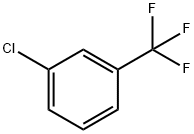
62-53-3
1423-26-3

101-23-5
To a stirred solution of deionized water (10 mL) containing aniline (1.0 mmol), 3-(trifluoromethyl)phenylboronic acid (1.0 mmol), and K2CO3 (2.0 mmol) was added an aqueous suspension of FePd nanowires (3.0 mol%, dissolved in 3 mL H2O) at room temperature. The reaction mixture was stirred continuously for 5 h at room temperature. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, 2 M HCl solution was added and the catalyst was separated using an external magnet. Subsequently, the catalyst was washed with ethyl acetate (EtOAc). The reaction mixture was extracted with ethyl acetate (2 × 20 mL), and the organic phases were combined, dried over anhydrous sodium sulfate and concentrated. Finally, the residue was purified by gel permeation chromatography to afford the target product N-phenyl-3-(trifluoromethyl)aniline.
Yield: 94%
Reaction Conditions:
with potassium carbonate in water at 20; for 5 h;
Steps:
Preparation of diphenylamine; typical procedure
General procedure: To a stirred solution of phenylboronic acid (1.0 mmol), aniline (1.0 mmol), and K2CO3 (2.0 mmol) in deionized H2O (10 mL) at room temperature was added an aqueous suspension of FePd nanowires (3.0 mol % in 3 mL of H2O). The mixture was stirred at room temperature for 5h. After completion of the reaction (as monitored by TLC), 2 M HCl was added and the catalyst was separated by applying an external magnet. The catalyst was washed with EtOAc. The mixture was extracted with EtOAc (2 * 20 mL), dried, and concentrated. The residue was subjected to gel permeation chromatography to afford pure product.
References:
Nasrollahzadeh, Mahmoud;Azarian, Abbas;Ehsani, Ali;Zahraei, Ali [Tetrahedron Letters,2014,vol. 55,# 17,p. 2813 - 2817]