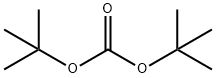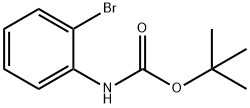
N-(TERT-BUTOXYCARBONYL)-2-BROMOANILINE synthesis
- Product Name:N-(TERT-BUTOXYCARBONYL)-2-BROMOANILINE
- CAS Number:78839-75-5
- Molecular formula:C11H14BrNO2
- Molecular Weight:272.14

24424-99-5
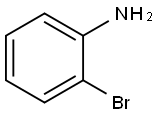
615-36-1

78839-75-5
The general procedure for the synthesis of N-(tert-butoxycarbonyl)-2-bromoaniline from di-tert-butyl dicarbonate and o-bromoaniline: di-tert-butyl dicarbonate (10.90 g, 50 mmol) was added to a solution of 2-bromoaniline (3.43 g, 20 mmol) in tetrahydrofuran (THF, 40 mL), followed by stirring of the reaction mixture for 3 days under reflux conditions. Upon completion of the reaction, the mixture was diluted by adding ether (50 mL), and the organic phase was washed sequentially with saturated sodium chloride solution (50 mL x 3) and dried with anhydrous magnesium sulfate (MgSO?). The solvent was removed by concentration under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography with the eluent of petroleum ether/ethyl acetate (20:1, v/v), and tert-butyl N-(2-bromophenyl)carbamate (4.73 g, 87% yield) was finally obtained.
36016-38-3
361 suppliers
$6.00/5g

78839-75-5
77 suppliers
$37.00/1g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: dicyclohexyl-carbodiimide / dichloromethane / 0.5 h / -15 °C
2: styrene; C8H24O19V6(2-)*2C19H27N4O2Pd(1+)*H2O; caesium carbonate / chlorobenzene / 1 h / 100 °C / Schlenk technique; Inert atmosphere
References:
Li, Peihe;Ma, Nuannuan;Li, Jikun;Wang, Zheng;Dai, Qipu;Hu, Changwen [Journal of Organic Chemistry,2017,vol. 82,# 15,p. 8251 - 8257]

24424-99-5
868 suppliers
$13.50/25G

615-36-1
415 suppliers
$10.00/5g

78839-75-5
77 suppliers
$37.00/1g
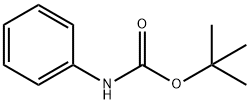
3422-01-3
105 suppliers
$10.00/1g

78839-75-5
77 suppliers
$37.00/1g

880384-47-4
11 suppliers
$371.00/1g

78839-75-5
77 suppliers
$37.00/1g