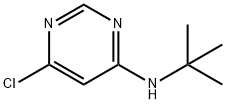
N-(tert-Butyl)-6-chloropyrimidin-4-amine synthesis
- Product Name:N-(tert-Butyl)-6-chloropyrimidin-4-amine
- CAS Number:945896-38-8
- Molecular formula:C8H12ClN3
- Molecular Weight:185.65
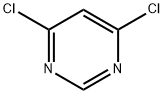
1193-21-1
575 suppliers
$16.00/5g
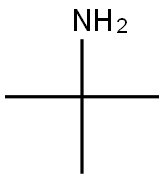
75-64-9
448 suppliers
$10.00/10g

945896-38-8
32 suppliers
$45.00/50mg
Yield:945896-38-8 90%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in isopropyl alcohol at 90; for 12 h;
Steps:
20.20a
Example 20 TV4 -tert-Butyl-N6-(4-methoxyphenyl)pyrimidine-4,6-diamine 20a. N-tert-Butyl-6-chloropyrimidin-4-amine 251 mg of 4,6-dichloropyrimidine , 123 mg of 2-methylpropan-2-amine and 260 mg of DIPEA were dissolved in 2.5 mL of i-PrOH and charged into a screw cap vial and the vial obtained was kept overnight in a heating block at 90°C. The reaction was monitored by TLC and was completed after 12 hours. The mixture obtained was cooled to r.t., solvent was evaporated and pure N-tert-butyl-6-chloropyrimidin-4-amine was obtained by column chromatography using PE: EtOAc 10: 1. Yield: 90% of theory. NMR (CDC13, 200MHz): δ = 1.43 (s, 9H), 4.93-5.19 (bs, 1H), 7.26 (s, 1H), 8.31 (s,1H).13C NMR (CDCb, 50MHz): 5 = 28.9 (q), 51.8 (s), 103.6 (d), 158.2 (d), 158.9 (s), 162.6 (s).
References:
WO2011/79343,2011,A2 Location in patent:Page/Page column 37-38