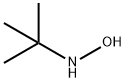
N-tert-butylhydroxylamine synthesis
- Product Name:N-tert-butylhydroxylamine
- CAS Number:16649-50-6
- Molecular formula:C4H11NO
- Molecular Weight:89.14
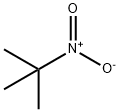
594-70-7
115 suppliers
$36.50/5g

16649-50-6
46 suppliers
$110.00/250mg
Yield: 100%
Reaction Conditions:
with acetic acid;zinc in ethanol at 10 - 20; for 3 h;
Steps:
2 Preparation of N-t-Butylhydroxylamine
1. A 500 mL three neck round bottom flask is equipped with a magnetic stir bar, thermometer adapter, thermometer, and addition funnel. 2. 95% ethanol (350 mL) was added to the flask and cooled to 10°C in an ice bath. (0121) 3. 2-Methyl-2-nitropropane (6.18 g, 0,060 mole), and zinc dust (5.89 g, 0,090 mole) were added in single portions. (0122) 4. Glacial acetic acid (10.8 g, 0,180 mole) was placed in the addition funnel and added dropwise at such a rate with vigorous stirring to maintain the temperature below 15°C. (0123) 5. The ice bath was removed and mixture was stirred for 3 hrs at room temperature. (0124) 6. The solvent was stripped from the mixture, leaving t-butylhydroxylamine, zinc acetate, and water. (0125) 7. Dichloromethane (50 mL) was added and the mixture filtered through a Buchner funnel. 8. The zinc acetate cake left on the filter paper was washed with 2X 25 mL dichloromethane. (0126) 9. Water was separated from the filtrate in a separatory funnel and the organic layer dried over magnesium sulfate. (0127) 10. The magnesium sulfate was removed by filtering through fluted filter paper, then dichloromethane stripped off by rotary evaporation. (0128) 11. The product (100% yield=5.34 g), a viscous liquid, was dissolved in methanol (50 mL) for use below.
References:
OKLAHOMA MEDICAL RESEARCH FOUNDATION;TOWNER, Rheal, A. WO2019/60152, 2019, A1 Location in patent:Page/Page column 21-22
121-32-4
739 suppliers
$5.00/10g

638-45-9
165 suppliers
$6.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

16649-50-6
46 suppliers
$110.00/250mg
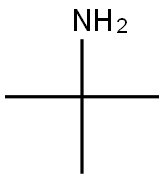
75-64-9
448 suppliers
$10.00/10g

16649-50-6
46 suppliers
$110.00/250mg

594-70-7
115 suppliers
$36.50/5g

16649-50-6
46 suppliers
$110.00/250mg

75-64-9
448 suppliers
$10.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

16649-50-6
46 suppliers
$110.00/250mg