
Naphthalene, 1-(3-bromopropoxy)- synthesis
- Product Name:Naphthalene, 1-(3-bromopropoxy)-
- CAS Number:3351-50-6
- Molecular formula:C13H13BrO
- Molecular Weight:265.15
Yield:3351-50-6 91.6%
Reaction Conditions:
Stage #1: α-naphtholwith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.25 h;Inert atmosphere;
Stage #2: 1,3-dibromo-propane in N,N-dimethyl-formamide at 40; for 6 h;Inert atmosphere;
Steps:
3-Naphthoxypropyl bromide (1a) was synthesized accordingto Ref [24]. (Scheme 1). Under nitrogen atmosphere,1-naphthol (5.0 g, 34.7 mmol) and 7.2 g (52.2 mmol) ofpotassium carbonate in 30 mL dried dimethyl formamide(DMF) were stirred for 15 min at room temperature andthe solution of 8.3 g (41.6 mmol) 1,3-dibromopropane and40 mL DMF was added dropwise to the reaction system.The ensuing mixture was heated with stirring at 40 °C for6 h, then poured into water. The organic layer was extractedwith dichloromethane, dried over sodium sulfate. Columnchromatography (silica gel/petroleum ether/ethyl acetate)followed by usual workups gave the product 1a. Yield:8.4 g (91.6%) as pale yellow liquid.The synthesis of 1b-1d was carried out in a similar way.3-Naphthoxypropyl bromide (1a) Yield: 91.6%; pale yellowliquid. 1H NMR (500 MHz, CDCl3)δ, ppm 2.42 (m,2 H, -CH2), 3.89 (t, J = 6.3 Hz, 2H, -CH2Br), 4.33 (t,J = 5.9 Hz, 2H, -OCH2), 6.86 (m, 1H, Naph), 7.41(m, 1H,Naph), 7.48 (m, 1H, Naph), 7.53 (m, 2H, Naph), 7.85 (m,1H, Naph), 8.28 (m, 1H, Naph). MS: m/z 215.1 ([M + H]+)amu
References:
Chen, Shaorui;Shen, Fengjuan [Journal of Inclusion Phenomena and Macrocyclic Chemistry,2017,vol. 88,# 3-4,p. 229 - 238]
20009-25-0
15 suppliers
inquiry

3351-50-6
9 suppliers
inquiry
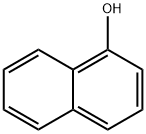
90-15-3
743 suppliers
$9.00/5g

3351-50-6
9 suppliers
inquiry

54804-70-5
6 suppliers
inquiry

3351-50-6
9 suppliers
inquiry

