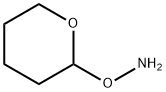
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine synthesis
- Product Name:O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine
- CAS Number:6723-30-4
- Molecular formula:C5H11NO2
- Molecular Weight:117.15
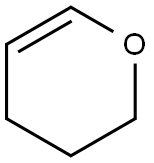
110-87-2

6723-30-4
General procedure for the synthesis of O-(tetrahydro-2H-pyran-2-yl)hydroxylamine from 3,4-dihydro-2H-pyran: N-hydroxyphthalimide (10 g, 0.061 mol) and 3,4-dihydro-2H-pyran (6.1 g, 0.073 mol) were added to a 250 mL three-necked flask, and tetrahydrofuran (100 mL) was added to dissolve. Subsequently, p-toluenesulfonic acid (1.1 g, 6.1 mmol) was added to catalyze the reaction. The reaction was carried out at room temperature for 2 hours. Upon completion of the reaction, the tetrahydrofuran was removed by distillation under reduced pressure. Water (200 mL) was added to the residue and extracted with dichloromethane (3 x 200 mL). The organic phases were combined and washed sequentially with saturated aqueous NaHCO3 solution (3 x 200 mL) and saturated aqueous NaCl solution (200 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and evaporated to dryness to give a yellow-green solid (15.3 g). The resulting solid was transferred to a 1000 mL three-necked flask and ethanol (450 mL) was added and stirred to dissolve. 80% hydrazine hydrate (10.4 mL, 0.17 mol) was slowly added dropwise and the reaction was carried out for 1 hour at room temperature. The reaction solution was directly filtrated and the filtrate was evaporated to give the crude product (10.6 g). The crude product was dissolved with ethyl acetate (500 mL), mixed thoroughly and vacuum filtered to remove insoluble matter. The filtrate was washed with water (3×100 mL) and saturated NaCl aqueous solution (200 mL) sequentially, dried with anhydrous sodium sulfate and filtered, evaporated to dryness to obtain a light yellow oil. The oily material was dried under vacuum to give a yellow solid (5.39 g) with a melting point of 35-37 °C and a yield of 75.3%.

110-87-2
544 suppliers
$10.00/1g

6723-30-4
203 suppliers
$6.00/1g
Yield:6723-30-4 75.3%
Reaction Conditions:
Stage #1:3,4-dihydro-2H-pyran with N-hydroxyphthalimide;toluene-4-sulfonic acid in tetrahydrofuran at 20; for 2 h;
Stage #2: with hydrazine in ethanol at 20; for 1 h;
Steps:
1 Synthesis of O-(tetrahydro-2H-pyran-2-yl)hydroxylamine (BXH-2)
A 250 mL three-necked flask was charged with N-hydroxyphthalimide (10 g, 0.061 mol)Dihydropyran (6.1 g, 0.073 mol),Tetrahydrofuran 100mL,After dissolution, p-toluenesulfonic acid (1.1 g, 6.1 mmol) was added,Reaction at room temperature 2h.The tetrahydrofuran was distilled off under reduced pressure,To the residue was added 200 mL of water,Dichloromethane 3 × 200mL extraction,Saturated NaHCO3 aqueous solution 3 × 200mL wash,Saturated NaCl aqueous solution 200mL washed once,Anhydrous sodium sulfate, filtration, evaporated to dryness,A yellow-green solid, 15.3g.The resulting solid was added to a 1000 mL three-necked flask,Ethanol 450mL, stirred to dissolve,80% hydrazine hydrate (10.4 mL, 0.17 mol) was added dropwise, Reaction at room temperature 1h.The reaction solution was directly suction filtered,The filtrate evaporated to give crude product 10.6g, was added ethyl acetate 500mL,After full mixing, Vacuum filtration insoluble matter,The filtrate was washed with water 3 × 100mL,Saturated brine solution 200mL washed once,Anhydrous sodium sulfate, filtration, evaporated to dryness,Had pale yellow oil, vacuum-dried yellow solid 5.39g,m.p. 35-37 ° C, yield 75.3%.
References:
Shenyang Pharmaceutical University;Chen Guoliang;Bao Xuefei;Zhou Qifan;Liu Ziao;Gao Xun CN107400131, 2017, A Location in patent:Paragraph 0031; 0063-0065
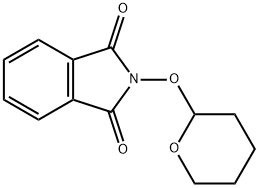
6584-60-7
4 suppliers
inquiry

6723-30-4
203 suppliers
$6.00/1g