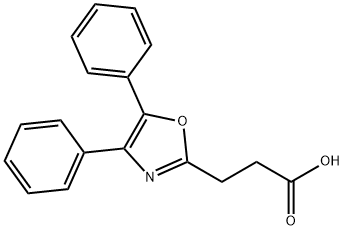
Oxaprozin synthesis
- Product Name:Oxaprozin
- CAS Number:21256-18-8
- Molecular formula:C18H15NO3
- Molecular Weight:293.32
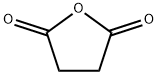
108-30-5
681 suppliers
$5.00/25g
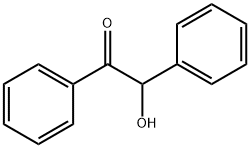
119-53-9
412 suppliers
$5.00/25g

21256-18-8
288 suppliers
$5.00/50mg
Yield:21256-18-8 67%
Reaction Conditions:
with pyridine;ammonium acetate in water;acetic acid
Steps:
1 EXAMPLE 1
EXAMPLE 1 A 5 liter flask was set up and equipped with stirrer, thermometer, reflux condenser and nitrogen inlet. Succinic anhydride (373 grams), benzoin (531 grams) and pyridine (300 milliliters; 296 grams) were introduced. The flask was purged with nitrogen and the reaction mixture heated to 90°-95° C. The mixture was stirred for one and a half hours at this temperature. Glacial acetic acid (1300 milliliters) and ammonium acetate (385 grams) were added to the reaction mixture and the mixture reheated to 90°-95° C. Stirring was continued and this temperature was maintained for a further 2 hours. The mixture was filtered, rinsing through with glacial acetic acid (150 milliliters). Distilled water (750 milliliters) was added and the mixture reheated to 90°-95° C. before gradual cooling with stirring to 15° C. over 1 hour. After filtering and washing with a mixture of acetic acid and distilled water in a ratio of 2:1 (600 milliliters), the product was slurried with distilled water (1000 milliliters). After filtering and washing with distilled water (2*400 milliliters), the product was dried at 90°-100° C. under vacuum. 702 Grams (67% yield) of β-(4,5-diphenyloxazol-2-yl)propionic acid were obtained as a white crystalline powder, m.p. 163°-165° C. The material was investigated for quality by TLC. The product had high purity and merely showed a number of very faint trace impurities.
References:
John Wyeth & Brother Limited US4190584, 1980, A
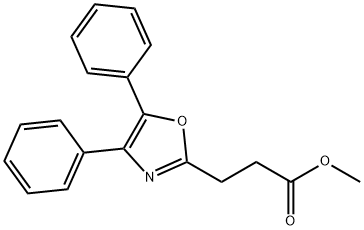
26820-94-0
1 suppliers
inquiry

21256-18-8
288 suppliers
$5.00/50mg
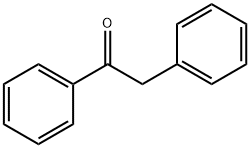
451-40-1
376 suppliers
$13.43/1gm:

21256-18-8
288 suppliers
$5.00/50mg
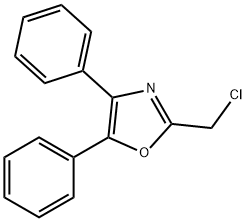
33161-99-8
0 suppliers
inquiry

21256-18-8
288 suppliers
$5.00/50mg
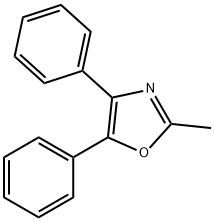
14224-99-8
44 suppliers
$90.00/500mg

21256-18-8
288 suppliers
$5.00/50mg