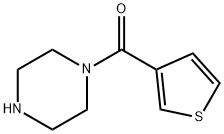
Piperazin-1-yl-thiophen-3-yl-methanone synthesis
- Product Name:Piperazin-1-yl-thiophen-3-yl-methanone
- CAS Number:59939-74-1
- Molecular formula:C9H12N2OS
- Molecular Weight:196.27
Yield:59939-74-1 67%
Reaction Conditions:
with benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate;benzotriazol-1-ol;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20;Inert atmosphere;
Steps:
General procedure for the synthesis of monoacylated diamines.
General procedure: In a typical procedure, alkanediamine (0.014 mol) was suspended in 20 ml DMF. Diisopropylethylamine (DIEA, 0.48 ml, 2.78 mmol) was added, followed by the appropriate carboxylic acid (1.39 mmol), 1-Hydroxybenzotriazole (HOBt, 380 mg, 2.78 mmol) and PyBOP (720 mg, 1.39 mmol). The reaction was allowed to proceed at room temperature 24-30 hours. DMF was then removed by evaporation under reduced pressure and the resultant residue was suspended in ethyl acetate and treated with an aqueous saturated solution of NaHCO3. Phases were separated and the aqueous one extracted with ethyl acetate. The organic layers were dried over magnesium sulfate and rotary evaporated to produce a crude yellow oil, which was purified by column chromatography (silica, CH2Cl2/MeOH 4:1).
References:
Moulat, Laure;Martinez, Jean;Salom-Roig, Xavier J. [Arkivoc,2019,vol. 2019,# 6,p. 336 - 349]
![tert-Butyl 4-[(thiophen-3-yl)carbonyl]piperazine-1-carboxylate](/CAS/20210111/GIF/1420959-59-6.gif)
1420959-59-6
10 suppliers
inquiry

59939-74-1
27 suppliers
inquiry
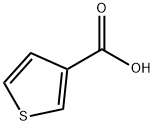
88-13-1
374 suppliers
$5.00/1g

59939-74-1
27 suppliers
inquiry
