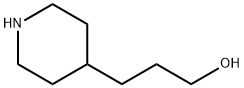
piperidine-4-propanol synthesis
- Product Name:piperidine-4-propanol
- CAS Number:7037-49-2
- Molecular formula:C8H17NO
- Molecular Weight:143.23
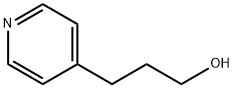
2629-72-3

7037-49-2
Platinum(IV) oxide (1.45 g, 6.4 mmol) was added to a mixed solution of 4-pyridinepropanol (10.0 g, 72.89 mmol) in methanol (110 mL) and 32% hydrochloric acid (18 mL) under argon protection. The reaction mixture was stirred vigorously under low pressure hydrogen (8 kPa) for 46 hours. Upon completion of the reaction, most of the catalyst was removed by filtration, followed by distillation under reduced pressure to remove the volatile solvent. The oily residue obtained was dissolved in 15% NaOH aqueous solution (80 mL) and extracted with dichloromethane (first 150 mL, then 3 x 100 mL). The organic phases were combined, washed with deionized water (20 mL) and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure and dried under vacuum to give 3-(4-piperidinyl)-1-propanol (10.3 g, 98% yield) as a white crystalline solid with a melting point of 58-60 °C. Thin layer chromatography (TLC) showed Rf = 0.2 (Expander: dichloromethane/methanol/28% ammonia = 50:10:1). Infrared spectroscopy (Nujol) showed characteristic absorption peaks located at 3290 cm?1 and 1320 cm?1. NMR hydrogen spectra (300 MHz, CD?OD) δ (ppm): 1.08-1.23 (m, 2H), 1.27-1.36 (m, 2H), 1.36-1.49 (m, 1H), 1.53-1.63 (m, 2H). 1.74 (br d, 2H, J≈13.4 Hz), 2.59 (dt, 2H, J=12.4, 2.6 Hz), 3.04 (td, 2H, J=12.4, 2.9 Hz), 3.56 (t, 2H, J=6.6 Hz). NMR carbon spectrum (75 MHz, CD?OD) δ (ppm): 31.5, 34.8, 35.2, 38.0, 47.9, 64.0. mass spectrum (ESI, MeOH) m/z (%): 287 (36) [2M+H]? , 144 (100) [M+H]? Molecular formula C?H??NO (molecular weight 143.2).

2629-72-3
146 suppliers
$10.00/1g

7037-49-2
135 suppliers
$19.00/250mg
Yield:7037-49-2 98%
Reaction Conditions:
with hydrogenchloride;Adam’s catalyst;hydrogen in methanol;water under 60.006 Torr; for 46 h;Inert atmosphere;
Steps:
8 4.1.8
4-(3-Hydroxypropyl)piperidine (17)
Under an atmosphere of argon platinum(IV)oxide (1.45 g, 6.4 mmol) was added to a solution of 16 (10.0 g, 72.89 mmol) in MeOH (110 mL) and 32% hydrochloric acid (18 mL).
The mixture was vigorously stirred under a low pressure of hydrogen (8 kPa) for 46 h.
The major part of the catalyst was removed by filtration and the volatiles were removed under reduced pressure.
The oily residue was taken up in 15% aq NaOH (80 mL) and the product was extracted with CH2Cl2 (150 and 3 * 100 mL).
The pooled extracts were washed with water (20 mL) and dried over Na2SO4.
Evaporation of the volatiles and drying in vacuo yielded product 17 as a white crystalline, compact solid (10.3 g, 98%) mp 58-60 °C. Rf = 0.2 (CH2Cl2/MeOH/28% aq NH3 50:10:1). IR (Nujol) 3290, 1320 cm-1. 1H NMR (300 MHz, CD3OD) δ (ppm) 1.08-1.23 (m, 2H), 1.27-1.36 (m, 2H), 1.36-1.49 (m, 1H), 1.53-1.63 (m, 2H), 1.74 (br d, 2H, J ca 13.4 Hz), 2.59 (dt, 2H, J 12.4 2.6 Hz), 3.04 (td, 2H, J 12.4 2.9 Hz), 3.56 (t, 2H, J 6.6 Hz).
13C NMR (75 MHz, CD3OD) δ (ppm) 31.5, 34.8, 35.2, 38.0, 47.9, 64.0. MS (ESI, MeOH) m/z (%) 287 (36) [2M+H]+, 144 (100) [M+H]+. C8H17NO (143.2).
References:
Keller, Max;Tränkle, Christian;She, Xueke;Pegoli, Andrea;Bernhardt, Günther;Buschauer, Armin;Read, Roger W. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 14,p. 3970 - 3990]
93524-95-9
37 suppliers
$40.00/250mg

7037-49-2
135 suppliers
$19.00/250mg
156185-63-6
131 suppliers
$24.00/250mg

7037-49-2
135 suppliers
$19.00/250mg
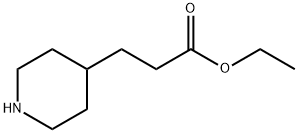
71879-55-5
35 suppliers
$190.00/500mg

7037-49-2
135 suppliers
$19.00/250mg
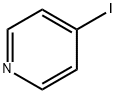
15854-87-2
314 suppliers
$6.00/1g

7037-49-2
135 suppliers
$19.00/250mg