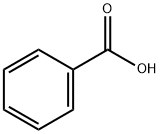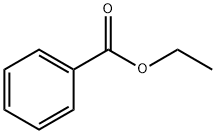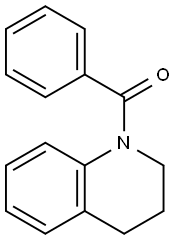
Quinoline, 1-benzoyl-1,2,3,4-tetrahydro- synthesis
- Product Name:Quinoline, 1-benzoyl-1,2,3,4-tetrahydro-
- CAS Number:16078-38-9
- Molecular formula:C16H15NO
- Molecular Weight:237.3
Yield:16078-38-9 95 %Chromat.
Reaction Conditions:
with triethylamine;2,2-dichloro-1,3-diisopropylimidazolidine-4,5-dione in dichloromethane at 20; for 3 h;Inert atmosphere;chemoselective reaction;
Steps:
General procedure for the synthesis of amides
General procedure: Carboxylic acid (1 mmol), amine or ammonium chloride (1 mmol) and triethylamine (3 or 4 mmol) were introduced to the solution of DCDD (1.05 mmol) and stirred at r.t. for 3 h. After reaction, the mixture was distilled under evacuation to remove CH2Cl2. Then, 15 mL of H2O was added to the residues and a solution pH value of 8.0 was set with potassium carbonate. Next, the mixture was extracted with ethyl acetate (25 mL × 3), and the organic layer was isolated and combined. The organic layer was washed with brine and dried over anhydrous MgSO4. Finally, the solvent was removed, and the residue was chromatographed on silica gel using hexane/ethyl acetate as eluent to give the corresponding pure product.
References:
Li, Jianhui;He, Shaopo;Fu, Haiqing;Chen, Xin;Tang, Min;Zhang, Dela;Wang, Bo [Research on Chemical Intermediates,2018,vol. 44,# 4,p. 2289 - 2303]
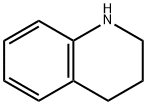
635-46-1
360 suppliers
$5.00/10g

60-29-7
0 suppliers
$31.19/50g
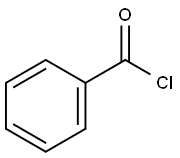
98-88-4
598 suppliers
$10.00/5g

16078-38-9
7 suppliers
inquiry
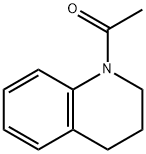
4169-19-1
63 suppliers
$60.00/500mg

16078-38-9
7 suppliers
inquiry