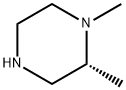
(R)-1,2-DIMETHYL-PIPERAZINE synthesis
- Product Name:(R)-1,2-DIMETHYL-PIPERAZINE
- CAS Number:623586-02-7
- Molecular formula:C6H14N2
- Molecular Weight:114.19

50-00-0
896 suppliers
$10.00/25g
163765-44-4
264 suppliers
$6.00/1g

623586-02-7
67 suppliers
$109.00/50mg
Yield:-
Reaction Conditions:
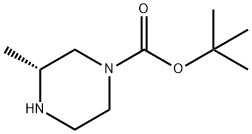
Stage #1:formaldehyd;(R)-tert-butyl 3-methylpiperazine-1-carboxylate with sodium cyanoborohydride;zinc(II) chloride in methanol;chloroform;water at 20;
Stage #2: with water;sodium hydrogencarbonate in methanol;chloroform
Stage #3: with trifluoroacetic acidmore than 3 stages;
Steps:
150.1
500 mg of t-butyl(3R)-3-methylpiperazin-1-carboxylate and 400 µL of formaldehyde solution (37%) were dissolved in 5 mL of chloroform, then a solution prepared by dissolving 151 mg of cyanotrihydro sodium borate and 163 mg of zinc chloride in 8 mL of methanol was added thereto, and stirred overnight at room temperature. The reaction mixture was diluted in 100 mL of chloroform, washed with a saturated aqueous solution of sodium hydrogen carbonate, and dried over anhydrous magnesium sulfate. The insolubles were filtered, the filtrate was concentrated under reduced pressure, and the obtained residue was dissolved in 4 mL of chloroform. Thereto, 4 mL of trifluoroacetic acid was added, and stirred at room temperature for 1 hour and a half. The reaction solution was concentrated under reduced pressure, the obtained residue was dissolved in methanol, and the trifluoroacetic acid was removed through a weak anion-exchange resin. The solvent was distilled off under reduced pressure, and 46 mg of (2R)-1,2-dimethylpiperazine [150-1] was obtained as a brown oily product.
References:
BANYU PHARMACEUTICAL CO., LTD. EP1790650, 2007, A1 Location in patent:Page/Page column 94