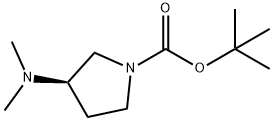
(R)-1-BOC-3-DIMETHYLAMINOPYRROLIDINE synthesis
- Product Name:(R)-1-BOC-3-DIMETHYLAMINOPYRROLIDINE
- CAS Number:1004538-33-3
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
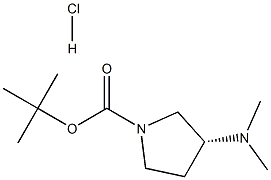
1004538-32-2

1004538-33-3
Step 9-2: Preparation of (R)-1-(tert-butoxycarbonyl)-3-dimethylaminopyrrolidine. 1.25 g of (R)-1-(tert-butoxycarbonyl)-3-dimethylaminopyrrolidine hydrochloride (chemical purity: 99.9 area %) was dissolved in a mixed system of 2.46 g of 23 wt% brine, 5.03 g of toluene, and 738 mg of 30 wt% aqueous sodium hydroxide, and the reaction mixture was stirred thoroughly. After completion of the reaction, liquid-liquid separation was carried out and the organic phase was collected. The organic phase was concentrated under reduced pressure to remove the solvent, followed by vacuum drying to afford 999 mg of (R)-1-Boc-3-(dimethylamino)pyrrolidine as a colorless transparent liquid (chemical purity: 99.5 area %, 95% yield). The structure of the product was confirmed by 1H-NMR (CDCl3): δ (ppm) 1.46 (s, 9H), 1.74 (m, 1H), 2.05 (m, 1H), 2.24 (s, 6H), 2.63 (m, 1H), 3.05 (m, 1H), 3.27 (m, 1H), 3.45-3.69 (m, 2H).

1004538-32-2
4 suppliers
inquiry

1004538-33-3
32 suppliers
$45.00/10mg
Yield: 95%
Reaction Conditions:
with sodium hydroxide;water in toluene
Steps:
9-2
Step 9-2: Production of (R)-1-(tert-butoxycarbonyl)-3-dimethylaminopyrrolidine To 1.25 g of (R)-1-(tert-butoxycarbonyl)-3-dimethylaminopyrrolidine hydrochloric acid salt (chemical purity: 99.9 area%) obtained in the step 9-1 were added 2.46 g of a 23% by weight brine, 5.03 g of toluene and 738 mg of a 30% by weight aqueous sodium hydroxide solution, and the mixture was stirred. After conducting liquid separation, the organic layer was concentrated. Then vacuum drying was carried out to give the title compound as 999 mg of a colorless and transparent liquid (chemical purity: 99.5 area%, yield 95%). 1H-NMR (CDCl3): d (ppm) 1.46 (s, 9H), 1.74 (m, 1H), 2.05 (m, 1H), 2.24 (s, 6H), 2.63 (m, 1H), 3.05 (m, 1H), 3.27 (m, 1H), 3.45-3.69 (m, 2H)
References:
Kaneka Corporation EP2050735, 2009, A1 Location in patent:Page/Page column 21-22

50-00-0
896 suppliers
$10.00/25g
147081-49-0
340 suppliers
$10.00/1g

1004538-33-3
32 suppliers
$45.00/10mg