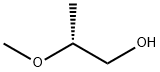
(R)-(-)-2-Methoxypropanol synthesis
- Product Name:(R)-(-)-2-Methoxypropanol
- CAS Number:6131-59-5
- Molecular formula:C4H10O2
- Molecular Weight:90.12
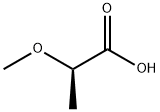
23943-96-6

6131-59-5
General procedure for the synthesis of (R)-2-methoxy-1-propanol from (R)-2-methoxypropionic acid:[1239] 858 μL (8.39 mmol, 1.8 eq.) of borane/dimethyl sulfide complex was slowly added to a solution containing 500 mg (4.66 mmol) of (R)-(+)-2-methoxypropionic acid under argon protection and at 0 °C. The reaction mixture was stirred overnight at room temperature and then 2M aqueous sodium hydroxide solution was added dropwise. After completion of the reaction, phase separation was carried out and the aqueous phase was extracted with dichloromethane. The organic phases were combined, dried over sodium sulfate, filtered, concentrated under reduced pressure (water bath temperature controlled at 300 mbar) and finally dried to give the product. Yield: 490 mg (quantitative). [1240] 1H-NMR (400 MHz, DMSO-d6): δ [ppm] = 4.55 (t, 1H), 3.40-3.31 (m, 1H), 3.30-3.20 (m, 2H), 3.24 (s, 3H), 1.02 (d, 3H).

23943-96-6
84 suppliers
$52.00/100mg

6131-59-5
41 suppliers
$503.87/5MG
Yield: 100%
Reaction Conditions:
with dimethylsulfide borane complex in dichloromethane at 0 - 20;Inert atmosphere;
Steps:
29.1A (2R)-2-Methoxypropan-1-ol
[1239] Under argon and at oo C., 858 fll (8.39 mmol, 1.8eq.) ofborane/dimethyl sulphide complex were added dropwiseto a solution of 500 mg ( 4.66 mmol) of (R)-( + )-2-methoxypropionic acid in 10 ml of dichloromethane, thereaction mixture was stirred at RT overnight and aqueoussodium hydroxide solution (2M) was then added dropwise.After phase separation, the aqueous phase was extracted withdichloromethane. The combined organic phases were dried(sodium sulphate), filtered, concentrated under reduced pressure(water bath 300 mbar) and dried.Yield: 490 mg (quant.)[1240] 1H-NMR (400 MHz, DMSO-d6): o [ppm]=4.55 (t,lH), 3.40-3.31 (m, lH), 3.30-3.20 (m, 2H), 3.24 (s, 3H), 1.02(d, 3H).
References:
BAYER PHARMA AKTIENGESELLSCHAFT;RÖHRIG, Susanne;HILLISCH, Alexander;STRAßBURGER, Julia;HEITMEIER, Stefan;SCHMIDT, Martina Victoria;SCHLEMMER, Karl-Heinz;TERSTEEGEN, Adrian;BUCHMÜLLER, Anja;GERDES, Christoph;SCHÄFER, Martina;KINZEL, Tom;TELLER, Henrik;SCHIROK, Hartmut;KLAR, Jürgen;NUNEZ, Eloisa JIMENEZ US2016/52884, 2016, A1 Location in patent:Paragraph 1238-1240
40105-20-2
22 suppliers
$339.00/1g

6131-59-5
41 suppliers
$503.87/5MG

67-56-1
790 suppliers
$7.29/5ml-f
16088-62-3
204 suppliers
$21.00/1mL
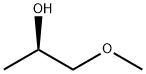
4984-22-9
151 suppliers
$8.00/1g

6131-59-5
41 suppliers
$503.87/5MG
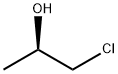
19141-39-0
90 suppliers
$14.00/250mg
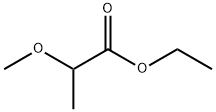
4324-39-4
14 suppliers
inquiry
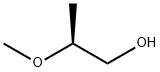
116422-39-0
73 suppliers
$739.77/1G

6131-59-5
41 suppliers
$503.87/5MG

67-56-1
790 suppliers
$7.29/5ml-f
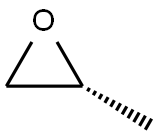
15448-47-2
198 suppliers
$50.00/1g
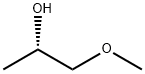
26550-55-0
132 suppliers
$8.00/1g

4984-22-9
151 suppliers
$8.00/1g

116422-39-0
73 suppliers
$739.77/1G

6131-59-5
41 suppliers
$503.87/5MG
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
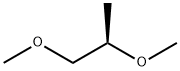
76946-22-0
0 suppliers
inquiry