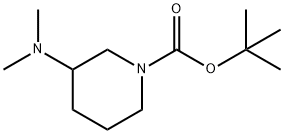
R-3-Dimethylamino-N-Boc-piperidine synthesis
- Product Name:R-3-Dimethylamino-N-Boc-piperidine
- CAS Number:1000576-83-9
- Molecular formula:C12H24N2O2
- Molecular Weight:228.33
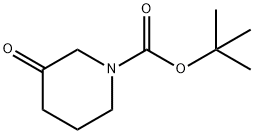
98977-36-7
414 suppliers
$6.00/1g

124-40-3
549 suppliers
$18.00/100ml

1000576-83-9
15 suppliers
inquiry
Yield:1000576-83-9 88%
Reaction Conditions:
Stage #1: 3-oxo-piperidine-1-carboxylic acid tert-butyl ester;dimethyl amine in tetrahydrofuran;dichloromethane at 20; for 0.0833333 h;
Stage #2: with sodium tris(acetoxy)borohydride in tetrahydrofuran;dichloromethane at 20;
Steps:
18.1 Step 1: tert-butyl 3-(dimethylamino)piperidinyl-1-carboxylate
To a solution of tert-butyl 3-oxopiperi (500 mg, 2.5 mmol) in dichloromethane (10 mL) was added a solution of dimethylamine in tetrahydrofuran (3.8 mL, 2 M, 7.5 mmol). After stirring at room temperature for 5 minutes, NaBH(OAc)3 (1.06 g, 5 mmol) was added to the mixture. The mixture was stirred at room temperature overnight then concentrated under vacuum and purified by silica gel column chromatography (CH2Cl2:MeOH = 10:1) to give tert-butyl 3-(dimethylamino)piperidinyl-1-carboxylate (500 mg, 88%) as a white solid. LCMS m/z = 229.0 [M+H]+.
References:
WO2017/216726,2017,A1 Location in patent:Page/Page column 555

98977-36-7
414 suppliers
$6.00/1g

1000576-83-9
15 suppliers
inquiry