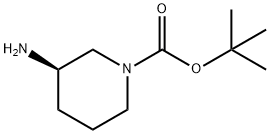
(R)-1-Boc-3-Aminopiperidine synthesis
- Product Name:(R)-1-Boc-3-Aminopiperidine
- CAS Number:188111-79-7
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28
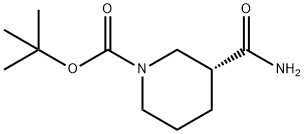
915226-43-6

188111-79-7
The general procedure for the synthesis of (R)-1- tert-butyl tert-butyloxycarbonyl-3-aminopiperidine from (R)-3-carbamoylpiperidine-1-carboxylic acid is as follows: in Example 28, (R)-1-(tert-butoxycarbonyl)-3-aminopiperidine was prepared as follows: firstly, (R)-1-(tert-butoxycarbonyl)piperidine amide (9 g of a solution, wherein the net weight of (R)-1 -(tert-butoxycarbonyl)piperidine amide is 0.7 g), sodium hydroxide (1.3 g, 10 equiv) and sodium hypochlorite aqueous solution (2.2 g, 10 equiv) are combined. Oxycarbonyl)piperidinamide has a net weight of 0.7 g), sodium hydroxide (1.3 g, 10 eq.) and aqueous sodium hypochlorite (2.2 g, 1.1 eq.) were mixed. The reaction mixture was stirred at 15 to 25°C for 16 hours. Upon completion of the reaction, the aqueous layer was separated and removed, followed by evaporation of the solvent under reduced pressure. Toluene (10 mL) and saturated brine (5 mL) were added to the residue and the aqueous layer was again separated and removed. Finally, the solvent was evaporated under reduced pressure to afford (R)-1-tert-butoxycarbonyl-3-aminopiperidine, the product was a yellow oil (0.7 g, net weight: 0.5 g, yield: 80%).
![1-Piperidinecarboxylic acid, 3-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, (3R)-](/CAS/GIF/320580-76-5.gif)
320580-76-5
42 suppliers
inquiry

188111-79-7
360 suppliers
$6.00/1g
Yield:188111-79-7 99%
Reaction Conditions:
Pd-C in methanol
Steps:
22 Synthesis of 3(R)-amino-piperidine-1-carboxylic Acid Tert-Butyl Ester
EXAMPLE 22 Synthesis of 3(R)-amino-piperidine-1-carboxylic Acid Tert-Butyl Ester To a slurry of Pd-C 10%, 170 mg) in MeOH (2 ml) was added a solution of the 3(R)-Benzyloxycarbonylamino-piperidine-1-carboxylic acid tert-butyl ester (1.0 g) in MeOH (60 ml). The mixture was stirred under H2 (1 atm) for 8 hr. After being filtered through Celite, the filtrate was evaporated to give a light yellow oil (572 mg, 99%). 1H-NMR (300 MHz, CDCl3) δ 4.00-3.78 (m, 2H), 3.80 (m, 2H), 3.60 (m, 1H), 1.90 (m, 1H), 1.70 (m, 1H), 1.60-1.40 (m, 12H), 1.30 (m, 1H) ppm; IR (film) 3485, 3361, 3292, 2975, 22931, 2862, 1692, 1608, 1548, 1479, 1427, 1367, 1266, 1242, 1162 cm-1; LRMS (calculated for C10,H20N2O2) 200, found 200.
References:
Aquila, Brian M.;Cuny, Gregory D.;Hauske, James R.;Shao, Liming;Wu, Xinhe US2001/56090, 2001, A1
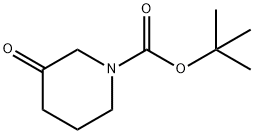
98977-36-7
410 suppliers
$6.00/1g

188111-79-7
360 suppliers
$6.00/1g

915226-43-6
43 suppliers
$29.00/250mg

188111-79-7
360 suppliers
$6.00/1g