
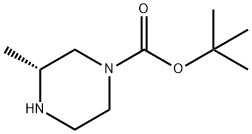
(R)-4-Boc-1-Cbz-2-methyl-piperazine synthesis
- Product Name:(R)-4-Boc-1-Cbz-2-methyl-piperazine
- CAS Number:1163793-25-6
- Molecular formula:C18H26N2O4
- Molecular Weight:334.41
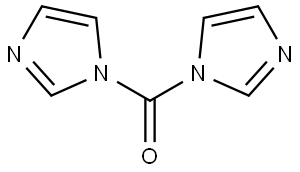
530-62-1
969 suppliers
$6.00/25g

100-51-6
1433 suppliers
$5.00/100g
163765-44-4
264 suppliers
$6.00/1g

1163793-25-6
6 suppliers
inquiry
Yield:1163793-25-6 53%
Reaction Conditions:
Stage #1: 1,1'-carbonyldiimidazole;benzyl alcoholwith triethylamine in tetrahydrofuran at 20; for 0.333333 h;Inert atmosphere;
Stage #2: (R)-tert-butyl 3-methylpiperazine-1-carboxylate in tetrahydrofuran at 75; for 26 h;Inert atmosphere;
Steps:
38.1
To a solution of 1, 1'-carbonyldiimidazole (1.0 g,5.99 mmol) in anhydrous THF (5 mL) was added triethylamine (0.42 mL, 3.00 mmol), and then added phenylmethanol (0.62 mL, 5.99 mmol)dropwise at rt. The mixture was stirred at rt for 20 min, and a solution of (R)-tert-butyl 3-methylpiperazine-1-carboxylate (300 mg, 1.50 mmol) in anhydrousTHF (9 mL) was added. The resulting mixture was stirred at 75 for 26 h, andacidized with dilute HCl (10 mL × 3) . The resulting mixture was extracted withEtOAc (15 mL × 2) . The combined organic layers were washed with saturatedaqueous sodium bicarbonate (10 mL × 2) , dried over anhydrous Na2SO4and concentrated. The residue was purified by silica gel chromatography elutedwith Petroleum ether/EtOAc (v/v) 5/1 to give (R) -1-benzyl 4-tert-butyl2-methylpiperazine-1, 4-dicarboxylate as colorless liquid (267 mg, 53) . 1H NMR (400 MHz, CDCl3): δ ppm 7.36-7.31 (m, 5H) , 5.14 (s, 2H) , 4.32 (br. s, 1H) , 4.15-3.97 (m, 1H), 3.91-3.83 (m, 2H) , 3.15-3.07 (m, 1H) , 3.01-2.82 (m, 2H) , 1.46 (s, 9H) ,1.16 (d, J 6.8 Hz, 3H) and MS-ESI: m/z 235.3 [M+H-100] + .
References:
WO2016/34134,2016,A1 Location in patent:Paragraph 00380

501-53-1
408 suppliers
$10.00/1g

163765-44-4
264 suppliers
$6.00/1g

1163793-25-6
6 suppliers
inquiry