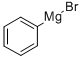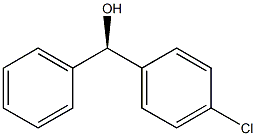
(R)-4-Chlorophenyl benzenemethanol synthesis
- Product Name:(R)-4-Chlorophenyl benzenemethanol
- CAS Number:123535-85-3
- Molecular formula:C13H11ClO
- Molecular Weight:218.68
Yield:93 % ee
Reaction Conditions:
Stage #1: phenylmagnesium bromide in tetrahydrofuran;1,4-dioxane; for 0.166667 h;
Stage #2: with triisopropoxytitanium(IV) chloride;(Ra)-5,6,7,8,5',6',7',8'-octahydro-[1,1']binaphthalenyl-2,2'-diol in tetrahydrofuran;1,4-dioxane;dichloromethane at 20; for 0.666667 h;
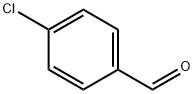
Stage #3: 4-chlorobenzaldehyde in tetrahydrofuran;1,4-dioxane;dichloromethane at 20; for 1.16667 h;
Steps:
16
Example 14 1.0 mL (12 mmol) of anhydrous 1,4-dioxane was added to 0.375 mL (0.375 mmol) of an anhydrous THF solution (1.00 mol/L) of commercial phenylmagnesium bromide, and the contents were stirred for 10 minutes, and then 0.402 mL (0.325 mmol) of an anhydrous dichloromethane solution (0.80 mol/L) of chloro-tri-iso-propoxy titanium prepared in Preparation Example 1 was added thereto, and further stirred for 10 minutes. 0.25 mL (0.0125 mmol) of an anhydrous dichloromethane solution (0.050 mol/L) of (R)-H8-BINOL was added thereto and stirred at room temperature for 30 minutes. The thus-obtained solution containing a white precipitate was added dropwise to 30 mg (0.25 mmol) of a dichloromethane solution (0.5 mL) of p-tolualdehyde using a syringe over a period of 10 minutes, and stirred at room temperature for 1 hour for the reaction. Thereafter, an operation was carried out in the same manner as in Example 1 to obtain (R)-4-methylbenzhydrol. The conversion rate of the raw material was 80%, while the enantiomeric excess of the product was 94 %ee. The amount of dioxane used during the reaction was 32 equivalents (64 equivalents in terms of the ether oxygen equivalent) relative to magnesiumExample 16 An operation was carried out in the same manner as in Example 14, except that 35 mg (0.25 mmol) of 4-chlorobenzaldehyde was used instead of p-tolualdehyde. Thus, (R)-4-chlorobenzhydrol was obtained. The conversion rate of the raw material was 61%, while the enantiomeric excess was 93 %ee.
References:
EP2412694,2012,A1 Location in patent:Page/Page column 22
104-88-1
646 suppliers
$5.00/25g

98-80-6
743 suppliers
$5.00/5g
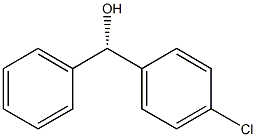
101402-04-4
10 suppliers
inquiry

123535-85-3
6 suppliers
inquiry
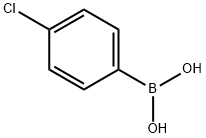
1679-18-1
624 suppliers
$10.00/1g

100-52-7
973 suppliers
$20.37/250g

101402-04-4
10 suppliers
inquiry

123535-85-3
6 suppliers
inquiry