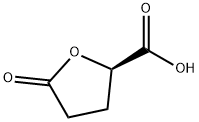
(R)-(-)-5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID synthesis
- Product Name:(R)-(-)-5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID
- CAS Number:53558-93-3
- Molecular formula:C5H6O4
- Molecular Weight:130.1
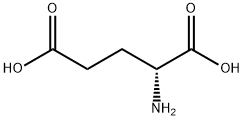
6893-26-1

53558-93-3
Example 1: Synthesis of (R)-(-)-5-oxo-2-tetrahydrofuran carboxylic acid (2) 1. To a mixed solution of D-glutamic acid (1 , 25 g, 170 mmol) in water (67 mL) and concentrated hydrochloric acid (35 mL) was slowly added a solution of NaNO2 (17.5 g, 253.6 mmol) in water (37.5 mL) at 0 °C for 4 hours. 2. After the addition was completed, the reaction mixture was stirred overnight at room temperature to obtain a clarified solution. 3. The solvent was removed by vacuum evaporation and the residue was treated with ethyl acetate (EtOAc, 80 mL) and filtered. 4. The filtrate was dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated. 5. The concentrated residue was crystallized from a solvent mixture of ethyl acetate/benzene/hexane to afford (R)-(-)-5-oxo-2-tetrahydrofuran carboxylic acid (2) as a white crystalline solid (12.63 g, 57% yield). Product Characterization: - Melting point: 71-73°C - 1H NMR (400 MHz, CD3OD): δ 4.20 (m, 1H, CHO), 1.8-2.3 (m, 4H, CH2CH2).
328-50-7
634 suppliers
$6.00/25g
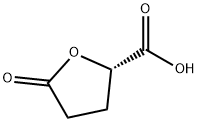
21461-84-7
200 suppliers
$19.00/250mg

53558-93-3
116 suppliers
$14.00/250mg
Yield:52 % ee
Reaction Conditions:
with nickel trifluoromethanesulfonate;1,2-bis((2S,5S)-2,5-diphenylphospholan-1-yl)ethane;2,2,2-trifluoroethanol;hydrogen at 65; under 38002.6 Torr; for 24 h;Schlenk technique;Overall yield = 95 percentChromat.; enantioselective reaction;
References:
Deng, Chen-Qiang;Deng, Jin;Fu, Yao;Gan, Li-Jin;Liu, Jiao;Luo, Jia-Hao [Angewandte Chemie - International Edition,2022,vol. 61,# 15,art. no. E202115983][Angew. Chem.,2022,vol. 134,# 15] Location in patent:supporting information

6893-26-1
480 suppliers
$6.00/10g

53558-93-3
116 suppliers
$14.00/250mg
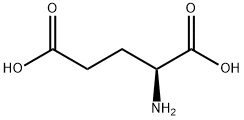
56-86-0
872 suppliers
$5.00/25g

53558-93-3
116 suppliers
$14.00/250mg