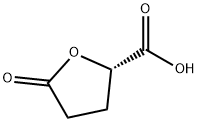
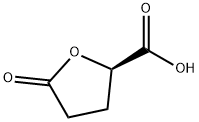
(S)-(+)-5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID synthesis
- Product Name:(S)-(+)-5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID
- CAS Number:21461-84-7
- Molecular formula:C5H6O4
- Molecular Weight:130.1
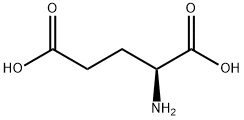
56-86-0

21461-84-7
General procedure for the synthesis of (S)-5-oxo-2-tetrahydrofuran carboxylic acid from L-glutamic acid: L-glutamic acid (10.07 g, 0.068 mol, JK CHEMICA) was dissolved in 20 mL of water in a conical flask. A solution of NaNO2 (7.0 g, 0.102 mol, Shantou Xilong Chemical Factory) in 20 mL of water was slowly added to this solution at -5°C, along with HCl and 40 mL of water. The reaction mixture was stirred continuously at room temperature for 12 hours. Subsequently, the reaction mixture was subjected to vacuum evaporation at less than 50 °C to obtain a yellow oily substance. This oily substance was dissolved in EtOAc, filtered to remove the solid formed and the solid was washed with EtOAc. The filtrate and washings were combined and dried with Na2SO4. Finally, the solvent was removed by vacuum concentration to afford (S)-5-oxo-2-tetrahydrofuran carboxylic acid as a light yellow oil (8.1 g, 91.6% yield). The product was analyzed by mass spectrometry (ESI, cation mode), m/z: 130.9 (M+1); 1H NMR (400 MHz, CDCl3) data were as follows: δ 2.27-2.41 (m, 1H), 2.44-2.65 (m, 3H), 5.09 (m, 1H), 9.12-9.55 (m, 1H).

56-86-0
872 suppliers
$5.00/25g

21461-84-7
201 suppliers
$19.00/250mg
Yield:21461-84-7 98%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water at 0 - 20;
Steps:
3.1. (S)-5-Oxotetrahydrofuran-2-carboxylic acid (6)
To the suspension of L-glutamic acid (10.0 g, 68 mmol) and NaNO2 (7.5 g, 108.7 mmol, 1.6 equiv) in water (100 mL) was added HCl (10 mL concentrated diluted to 50 mL) dropwise via an addition funnel at 0° C with vigorous stirringfor 1 h. The mixture was warmed to rt and stirred for 18 h. The water was evaporated and then EtOAc (200 mL) wasadded. The mixture was stirred for 30 min and filtered. The solid cake was washed with EtOAc (2 x 75 mL). The organic extracts were combined and dried over MgSO4. The solventwas evaporated in vacuo and the residue was left under high vacuum for 12 h to give the lactone acid 6 (8.67 g, 98% yield) as a solid syrup. 1H NMR (500 MHz, CDCl3) δ 5.01-4.97 (m, 1H), 2.68-2.53 (m, 3H), 2.44-2.35 (m, 1H). NMR spectroscopic data matched with the published data [12].
References:
Xuan, Duc Dau [Letters in Organic Chemistry,2021,vol. 18,# 1,p. 58 - 65]
21461-85-8
24 suppliers
inquiry

21461-84-7
201 suppliers
$19.00/250mg
328-50-7
634 suppliers
$6.00/25g

21461-84-7
201 suppliers
$19.00/250mg
53558-93-3
117 suppliers
$14.00/250mg
6893-26-1
480 suppliers
$6.00/10g

21461-84-7
201 suppliers
$19.00/250mg
13095-48-2
33 suppliers
$28.00/1mg

21461-84-7
201 suppliers
$19.00/250mg