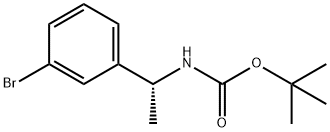
(R)-tert-butyl 1-(3-broMophenyl)ethylcarbaMate synthesis
- Product Name:(R)-tert-butyl 1-(3-broMophenyl)ethylcarbaMate
- CAS Number:1187932-25-7
- Molecular formula:C13H18BrNO2
- Molecular Weight:300.19

24424-99-5
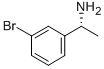
176707-77-0

1187932-25-7
General procedure for the synthesis of tert-butyl (R)-1-(3-bromophenyl)ethylcarbamate from di-tert-butyl dicarbonate and (R)-1-(3-bromophenyl)ethylamine: In Example 16, Compound 16a was synthesized as follows: (R)-1-(3-bromophenyl)ethylamine (1.023 g, 5.112 mmol) was dissolved in dichloromethane (20 mL), followed by addition of triethylamine (720 μL, 5.112 mmol) and di-tert-butyl dicarbonate (1.784 g, 8.179 mmol). The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the volatile solvent was removed by distillation under reduced pressure and the residue was purified by silica gel column chromatography using a 50 g Isolute column eluting with a continuous gradient of hexane/Et2O (1:0 to 4:1) to afford the title compound tert-butyl (R)-1-(3-bromophenyl)ethylcarbamate (1.552 g, 100%) as a white solid.1H NMR ( 300MHz, CDCl3) δ 1.43 (br s, 12H), 4.77 (br s, 2H), 7.16-7.26 (m, 2H), 7.39 (dt, J = 2.0,7.1Hz, 1H), 7.46 (s, 1H).

24424-99-5
869 suppliers
$13.50/25G

176707-77-0
93 suppliers
$40.00/1g

1187932-25-7
40 suppliers
$90.00/50mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20;
Steps:
16
Example 16, Compound 16; (E)-(2R,5S,11 S,14S,17R,18R)-11 -(3-Hydroxy-benzyl)-14-isopropyl-18-methoxy- 2,17-dimethyl-3,9,12,15,28-pentaaza-tricyclo[21.3.1.1 *5,9*]octacosa- 1 (27),21 ,23,25-tetraene-4,10,13,16-tetraone.; Com ound 16a: [(R)-1 -(3-Bromo-phenyl)-ethyl]-carbamic acid iert-butyl esterA solution of (R)-bromo-a-methylbenzylamine (1 .023 g, 5.1 12 mmol) indichloromethane (20 mL) was subsequently treated with triethylamine (720 μΙ_, 5.1 12 mmol) and di-te/t-butyl dicarbonate (1 .784 g, 8.179 mmol). After overnight stirring at room temperature, the volatiles were removed in vacuo and the residue was purified by silica gel chromatography using a 50 g Isolute cartridge eluted with a continuous gradient of /so-hexanes/Et20 1 :0 to 4:1 to afford the title compound (1 .552 g, 100%) as a white solid. H NMR (300 MHz, CDCI3) 1 .43 (br s, 12H), 4.77 (br s, 2H), 7.16-7.26 (m, 2H), 7.39 (dt, J = 2.0, 7.1 Hz, 1 H), 7.46 (s, 1 H).
References:
GILEAD SCIENCES, INC.;SELCIA LIMITED;APPLEBY, Todd;FLIRI, Hans, G.;KEATS, Andrew, J.;LAZARIDES, Linos;MACKMAN, Richard, L.;PETTIT, Simon, N.;POULLENNEC, Karine, G.;SANVOISIN, Jonathan;STEADMAN, Victoria, A.;WATT, Gregory, M. WO2012/78915, 2012, A1 Location in patent:Page/Page column 90