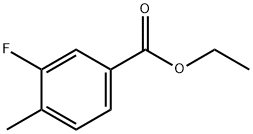
RARECHEM AL BI 0500 synthesis
- Product Name:RARECHEM AL BI 0500
- CAS Number:86239-00-1
- Molecular formula:C10H11FO2
- Molecular Weight:182.19

64-17-5
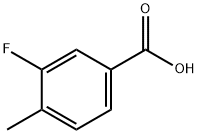
350-28-7

86239-00-1
Thionyl chloride (3 mL) was added slowly and dropwise to a solution of 3-fluoro-4-methylbenzoic acid (13.23 g, 85.85 mmol) in anhydrous ethanol (200 mL) at 0 °C. The reaction mixture was warmed to 60 °C and stirred overnight. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated under reduced pressure. The concentrate was diluted with dichloromethane and the resulting organic phase was washed sequentially with 1N sodium hydroxide solution (100 mL x 2). The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to give ethyl 3-fluoro-4-methylbenzoate (15.02 g, 96% yield).
Yield:86239-00-1 96%
Reaction Conditions:
with thionyl chloride at 0 - 60;
Steps:
To a solution of 3-fluoro-4-methylbenzoic acid (13.23 g, 85.85 mmol) in EtOH (200 mL) at 0 °C was added SOCl2 (3 mL) dropwise. The reaction mixture was heated at 60 °C overnight, then cooled to room temperature, concentrated under reduced pressure and diluted with DCM. The organic layer was washed with IN NaOH (100 mL x 2), dried (MgS04) and concentrated to give ethyl 3-fluoro-4-methylbenzoate (15.02 g, 96 %).
References:
WO2011/71565,2011,A1 Location in patent:Page/Page column 163