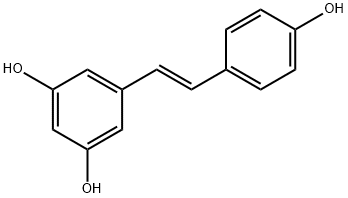
Resveratrol synthesis
- Product Name:Resveratrol
- CAS Number:501-36-0
- Molecular formula:C14H12O3
- Molecular Weight:228.24
89946-06-5
27 suppliers
inquiry

501-36-0
996 suppliers
$5.00/250mg
Yield:501-36-0 100%
Reaction Conditions:
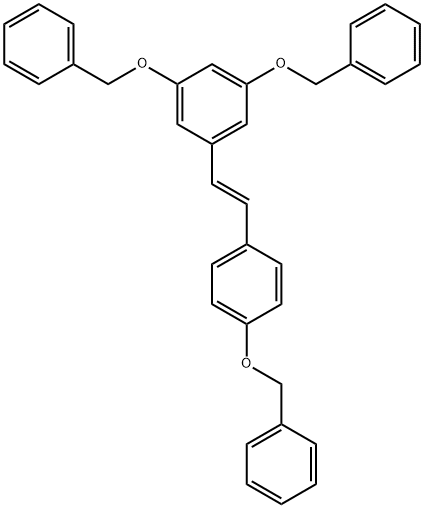
Stage #1: (E)-1-(4-(benzyloxy)phenyl)-2-(3,5-bis(benzyloxy)phenyl)-ethenewith aluminum (III) chloride;triethylamine in chlorobenzene at 0 - 80; for 10 h;
Stage #2: with water in chlorobenzene at 0; for 2 h;Product distribution / selectivity;
Steps:
3
4 ml of chlorobenzene and 6 g of triethylamine (59.3 mmol) are introduced into a three- necked round-bottomed flask. A nitrogen atmosphere is applied, the mixture is cooled to 0-50C and 4.9 g of anhydrous aluminium chloride (36.7 mmol) are introduced in small fractions at this temperature. The medium is brought to 500C and is maintained at this temperature for 1 h, and then 2 g of (E)-3,5,4'-tribenzyloxystilbene (4 mmol) dissolved in 5 ml of chlorobenzene are added in 1 h at this temperature. This temperature is maintained for 4 h and then a temperature of 80°C is maintained for 4 h. The mixture is brought back to ambient temperature, separation by settling is carried out and the heavy phase is recovered and poured onto 20 ml of a 50/50 ice/water mixture. The medium is kept stirred for 2 h and extracted with ethyl acetate. The organic phase is washed with a saturated aqueous sodium bicarbonate solution and then with water and then concentrated to result in 0.93 g of crude (E)-resveratrol, i.e. a practically quantitative yield with respect to the starting tribenzylresveratrol.
References:
WO2009/43761,2009,A1 Location in patent:Page/Page column 10
42206-94-0
232 suppliers
$6.00/1g

501-36-0
996 suppliers
$5.00/250mg
26153-38-8
273 suppliers
$20.00/250mg
![Phosphonic acid, [(4-hydroxyphenyl)methyl]-, dimethyl ester](/CAS/20180527/GIF/68997-88-6.gif)
68997-88-6
1 suppliers
inquiry

501-36-0
996 suppliers
$5.00/250mg
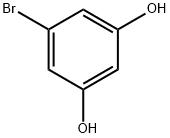
106120-04-1
149 suppliers
$8.00/100mg
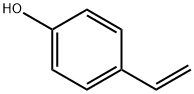
2628-17-3
263 suppliers
$6.00/1g

501-36-0
996 suppliers
$5.00/250mg
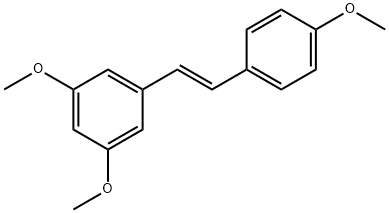
22255-22-7
214 suppliers
$5.00/50mg

501-36-0
996 suppliers
$5.00/250mg