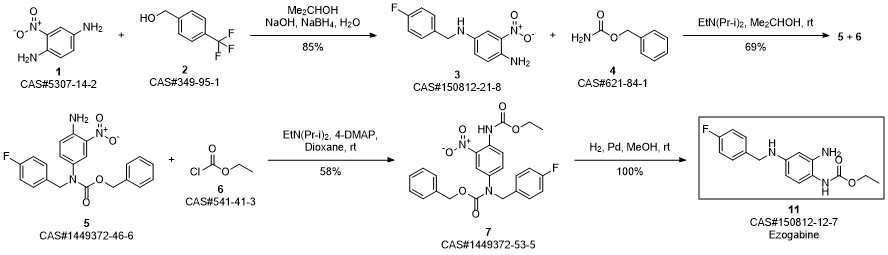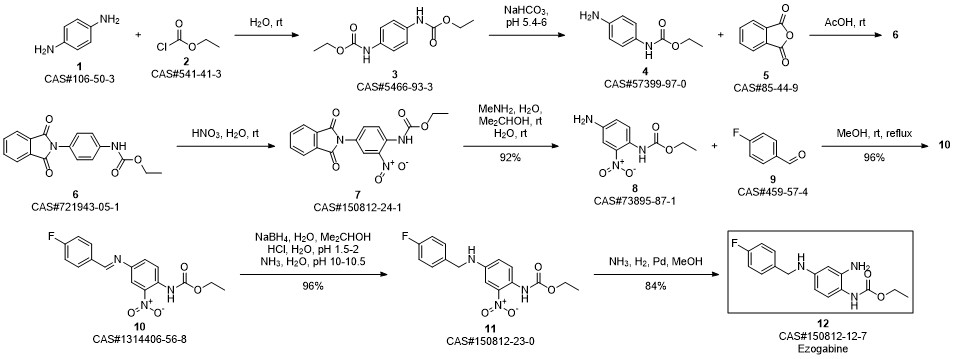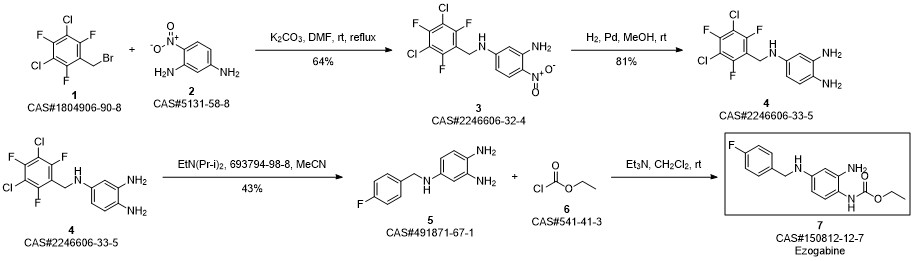
Retigabine synthesis
- Product Name:Retigabine
- CAS Number:150812-12-7
- Molecular formula:C16H18FN3O2
- Molecular Weight:303.33
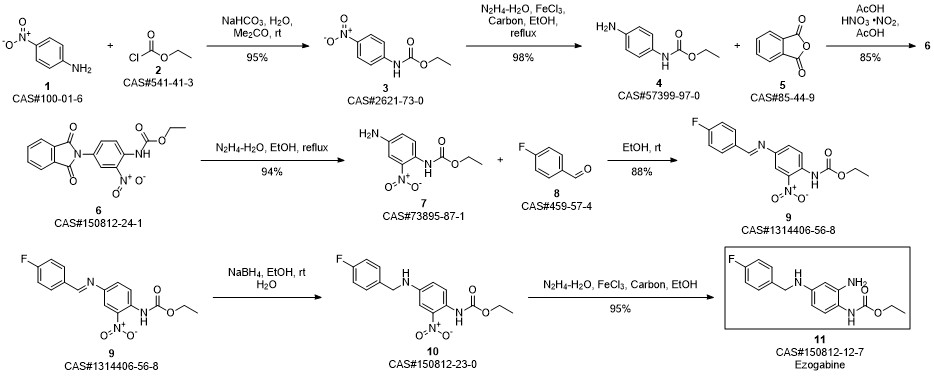
Zhu, Lei; Wang, Jia-le; Wang, Pu-hai. Improved synthesis of retigabine. Zhongguo Yaowu Huaxue Zazhi. Volume 24. Issue 1. Pages 31-33, 77. Journal. (2014).
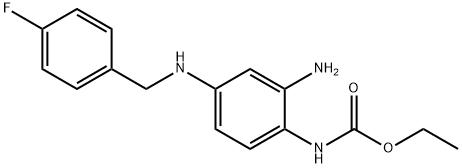
![{4-[(4-fluorobenzil)aMMino]-2-nitrofenil}carbaMMato di etile](/CAS/20150408/GIF/150812-23-0.gif)
150812-23-0
43 suppliers
inquiry

150812-12-7
242 suppliers
$39.00/1mg
Yield:150812-12-7 90%
Reaction Conditions:
with hydrogen;1 % platinum and 2% vanadium on carbon in ethanol at 50 - 70; under 1500.15 Torr; for 3 h;Inert atmosphere;Industry scale;
Steps:
3
A pressure vessel was charged with 2-ethyoxycarbonylamino-5-(4-fluorobenzylamino)- nitrobenzene, 1 kg (1 wt), and the catalyst, 1 % Pt + 2 % V/C, 50 g (0.05 wt). The vessel was pressure test with nitrogen to 6 barg. The reactor was charged with denatured ethanol, 10 L (10 vol), and the stir rate was set to > 450 rpm. The vessel was pressure purged 3 times with nitrogen to 2 barg. The reaction mixture was heated to 50 °C under reactor control. Once an internal temperature of 50 °C was achieved, agitation was discontinued and the reactor purged three times with hydrogen to 2 barg. Following the third hydrogen purge and once the vessel reached 2 barg again, hydrogen flow control was initiated and the agitator activated. The reactor contents were aged for 2 hours. The reaction was heated to 70 °C and stirred for an additional 1 hour at 70° C. Once complete, the reaction mixture was filtered. The filtrate was transfer to a second 20 L vessel. The reactor was rinsed with denatured ethanol, 3 L (3 vol) and heated to > 55 °C. The rinse was filtered and the solution transferred to the second 20-L vessel. Once the batch temperature dropped below 30 °C, a vacuum was established, 100 mbar (solution will boil at ~ 29 °C), and the solution concentrated to 7.5 L (7.5 vol). The solution was heated to 65 °C and aged until dissolution has occurred. The batch was cooled to 50 °C and seeded with retigabine (API), 5 g (0.005 wt) slurried in denatured ethanol, 20 ml_ (0.02 vol). After charging the seed, the solution was immediately cooled to 40 °C over 40 minutes, then aged for 60 min. The solution was cooled to 0 °C over 2 hours. The heterogeneous solution was stirred at 0 °C for 1 hour. The batch was milled, isolated and dried. The slurry was transferred to a filter and filtered. The wet cake was transferred to the vacuum oven and dried at 30-40 °C until the LOD indicated < 0.5 % wt. loss (120 °C for 15 minutes).Percent yield range observed: 70-90 %
References:
WO2012/98075,2012,A1 Location in patent:Page/Page column 8
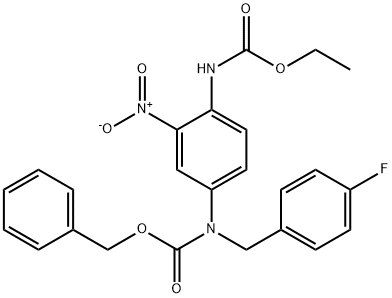
1449372-53-5
1 suppliers
inquiry

150812-12-7
242 suppliers
$39.00/1mg
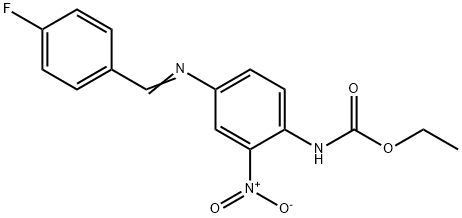
1314406-56-8
2 suppliers
inquiry

150812-12-7
242 suppliers
$39.00/1mg
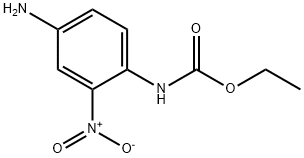
73895-87-1
29 suppliers
inquiry
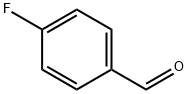
459-57-4
607 suppliers
$6.00/25g

150812-12-7
242 suppliers
$39.00/1mg
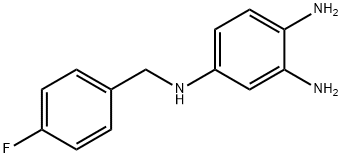
491871-67-1
24 suppliers
inquiry
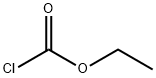
541-41-3
0 suppliers
$21.00/5g

150812-12-7
242 suppliers
$39.00/1mg