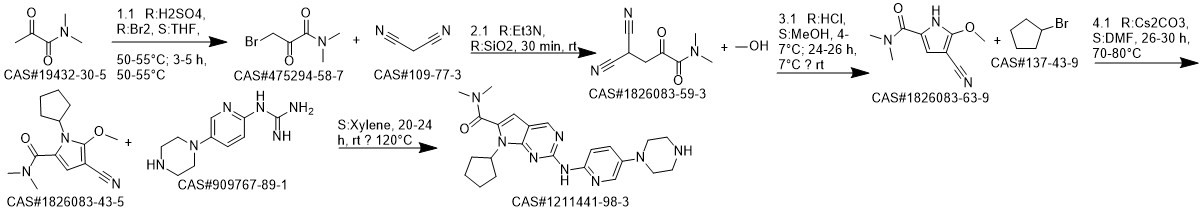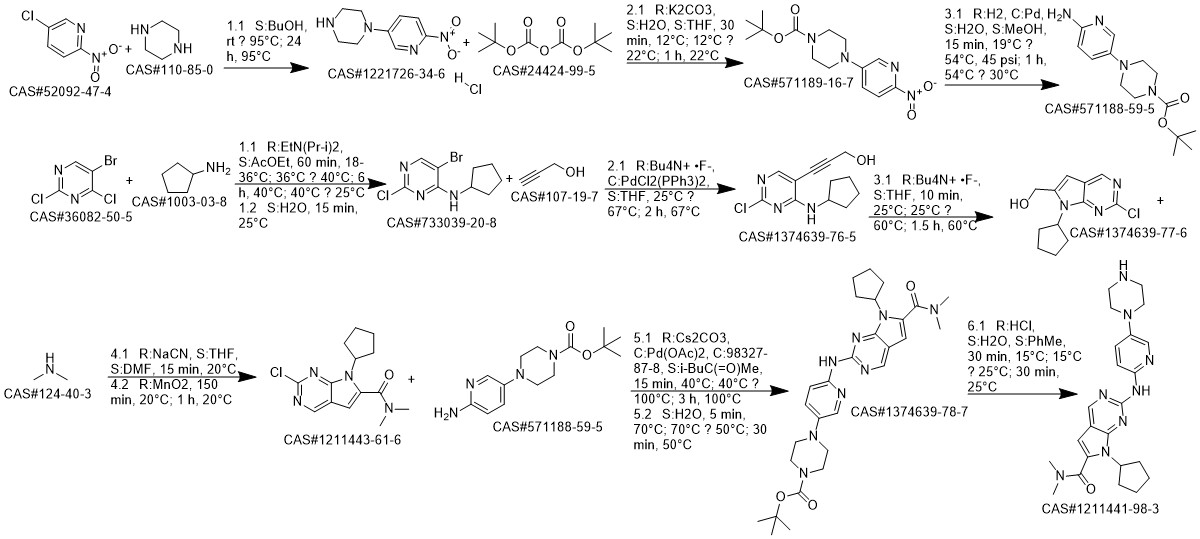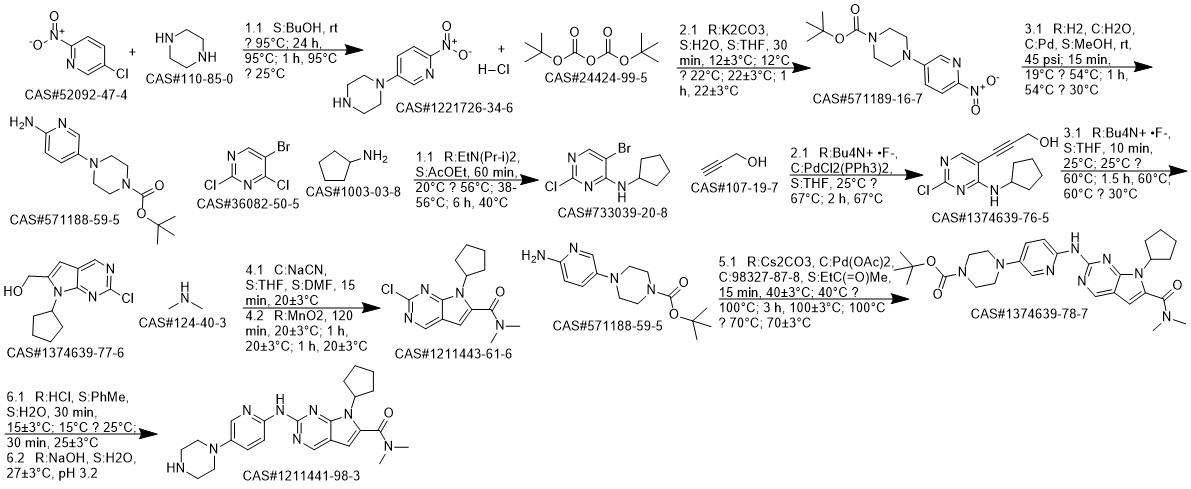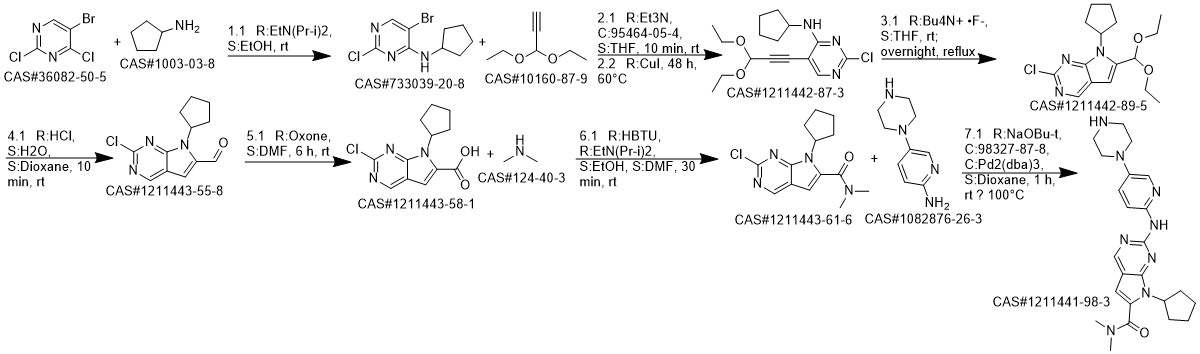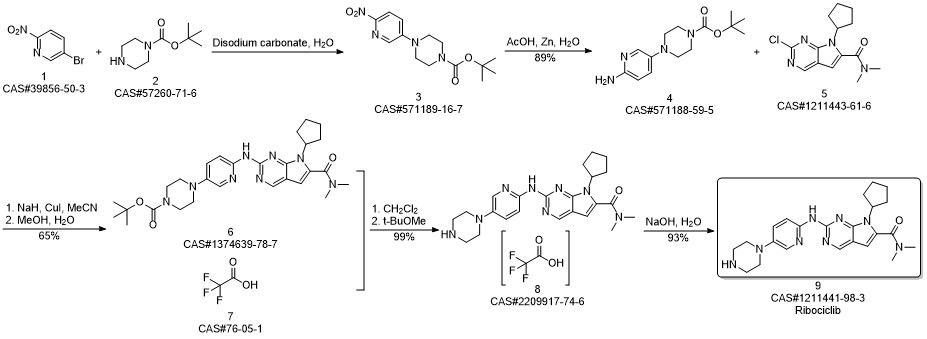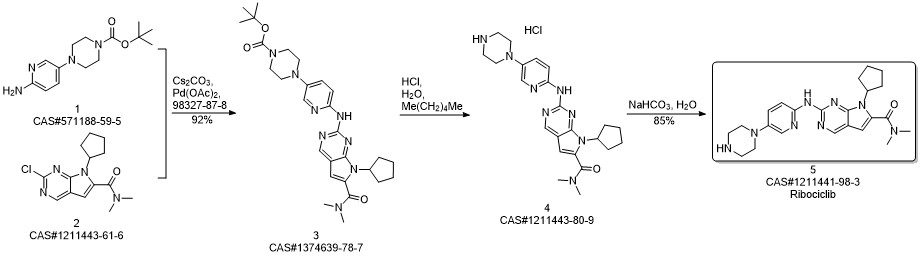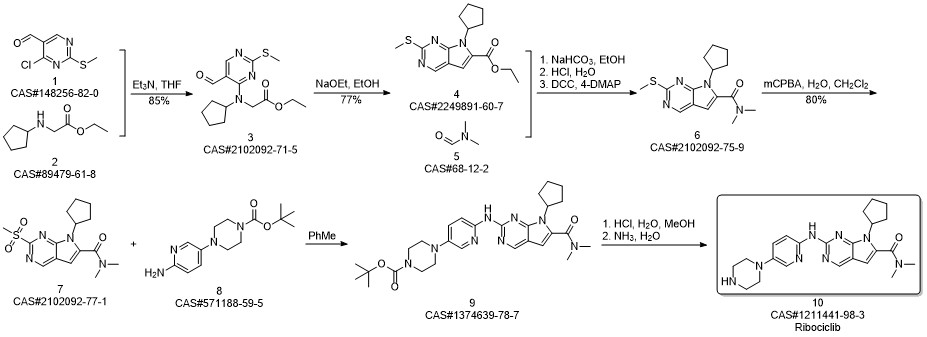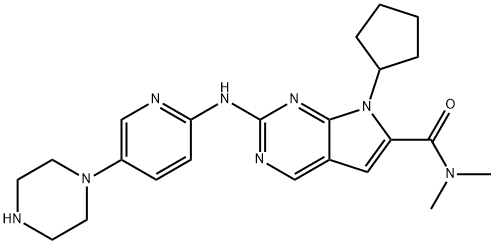
Ribociclib synthesis
- Product Name:Ribociclib
- CAS Number:1211441-98-3
- Molecular formula:C23H30N8O
- Molecular Weight:434.54
Reference: Reddy, Peddireddy Subba; Kumar, Kottur Mohan; Oruganti, Srinivas; Kandagatla, Bhaskar; Das Gupta, Shirshendu. Process for preparation of Ribociclib and its acid addition salts. WO 2018051280. (PCT Int. Appl. (2018))
![1-Piperazinecarboxylic acid, 4-[6-[[7-cyclopentyl-6-[(diMethylaMino)carbonyl]-7H-pyrrolo[2,3-d]pyriMidin-2-yl]aMino]-3-pyridinyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1374639-78-7.gif)
1374639-78-7
56 suppliers
inquiry

1211441-98-3
233 suppliers
$25.00/500μg
Yield:1211441-98-3 98.8%
Reaction Conditions:
with phosphoric acid in dichloromethaneReagent/catalyst;
Steps:
1.6 (6) Preparation of Ribociclib (X)
The above-obtained intermediate 7-cyclopentyl-N,N-dimethyl-2-{[5-(4-tert-butoxycarbonylpiperazin-1-yl)pyrazolePyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide (IX) (5.353 g, 10 mmol) is dissolved in 30 ml of dichloromethanThe alkane solution was added with 15 ml of hydrochloric acid (2 mol/L) and stirred at room temperature for 1 h. The reaction was stopped and then distilled under reduced pressure. The resulting concentrate was dissolved in 5 ml of ethyl acetate, and the organic phase was washed with saturated sodium bicarbonate to pH. 7-8, the aqueous phase was extracted twice with ethyl acetate, and the organic phases were combined, dried over anhydrous sodium sulfate, and distilled under reduced pressure. The resulting concentrate was recrystallized with n-hexane and vacuum dried to give Rebsini (a white solid product). X) 4.26 g; Yield 98.0%; Purity 99.8% (HPLC area normalization method); (6) Preparation of Rebocini (X)The difference with Example 1 is that the acid used is phosphoric acid.The final white solid product, rebamizin (X) 4.32 g; yield 98.8%; purity 99.8% (HPLC areaNormalization);
References:
Nanjing Qike Pharmaceutical Co., Ltd.;Wu Xueping;Xing Jigang;Chu Yijie;Yan Dongyang CN107936029, 2018, A Location in patent:Paragraph 0047; 0061-0062; 0078-0079; 0094-0096
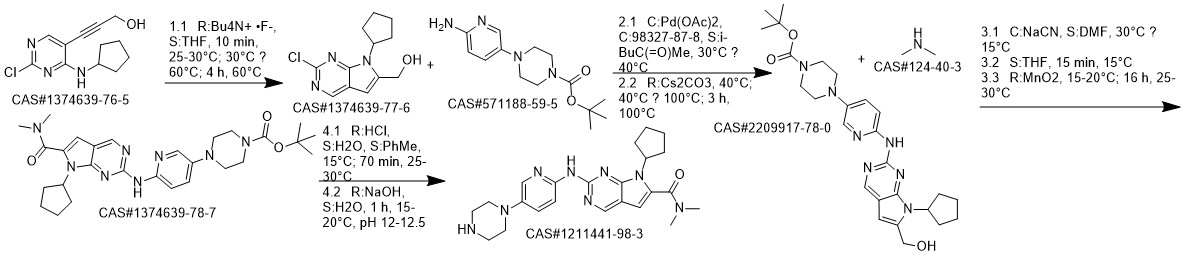
1003-03-8
231 suppliers
$10.00/1g

1211441-98-3
233 suppliers
$25.00/500μg
![2-Chloro-7-cyclopentyl-N,N-dimethyl-H-pyrrolo[2,3-d]pyrimidine-6-carboxamide](/CAS/20150408/GIF/1211443-61-6.gif)
1211443-61-6
200 suppliers
$18.00/250mg

1211441-98-3
233 suppliers
$25.00/500μg
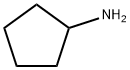
571188-59-5
539 suppliers
$13.00/1g

1211441-98-3
233 suppliers
$25.00/500μg
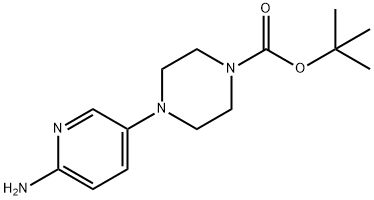
733039-20-8
275 suppliers
$6.00/1g

1211441-98-3
233 suppliers
$25.00/500μg