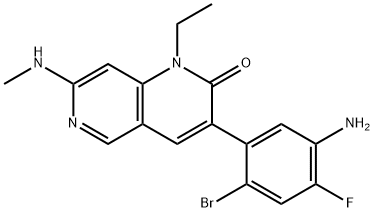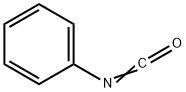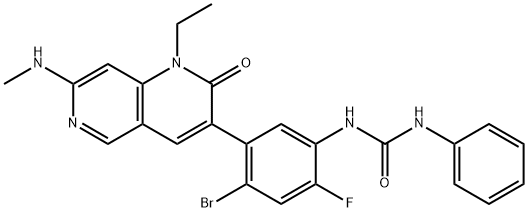
Ripretinib synthesis
- Product Name:Ripretinib
- CAS Number:1442472-39-0
- Molecular formula:C24H21BrFN5O2
- Molecular Weight:510.36

Yield:1442472-39-0 64.5%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 100 h;
Steps:
31 Example 31
A mixture of Example A14 (0.120 g, 0.307 mmol) and TEA (0.043 mL, 0.307 mmol) in THF (3.0 mL) was treated with phenyl isocyanate (0.040 g, 0.337 mmol) and stirred at RT for 4 h.
Over the course of the next 4 days the mixture was treated with additional phenyl isocyanate (0.056 mL) and stirred at RT.
The resulting solid was filtered, rinsed with THF, then triturated with MeOH to afford 1-(4-bromo-5-(1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2-fluorophenyl)-3-phenylurea (101 mg, 64.5% yield) as a bright white solid. 1H NMR (400 MHz, DMSO-d6): δ 9.09 (s, 1H), 8.68 (s, 1H), 8.41 (s, 1H), 8.17 (d, 1H), 7.70 (s, 1H), 7.65 (d, 1H), 7.41 (d, 2H), 7.27 (m, 2H), 7.03 (m, 1H), 6.96 (t, 1H), 6.23 (s, 1H), 4.13 (q, 2H), 2.86 (d, 3H), 1.20 (t, 3H); MS (ESI) m/z: 510.1 [M+H]+.
References:
Deciphera Pharmaceuticals, LLC;Flynn, Daniel L.;Kaufman, Michael D.;Petillo, Peter A. US8461179, 2013, B1 Location in patent:Page/Page column 85

959162-97-1
22 suppliers
inquiry

1442472-39-0
119 suppliers
inquiry
959162-99-3
31 suppliers
inquiry

1442472-39-0
119 suppliers
inquiry
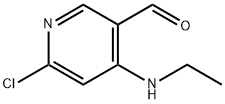
959163-01-0
50 suppliers
inquiry

1442472-39-0
119 suppliers
inquiry
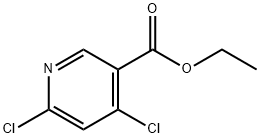
40296-46-6
323 suppliers
$6.00/1g

1442472-39-0
119 suppliers
inquiry