Regorafenib synthesis
- Product Name:Regorafenib
- CAS Number:755037-03-7
- Molecular formula:C21H15ClF4N4O3
- Molecular Weight:482.82

Du, Fangyu; Zhou, Qifan; Shi, Yajie; Yu, Miao; Sun, Wenjiao; Chen, Guoliang. A new pathway via intermediate 4-amino-3-fluorophenol for the synthesis of regorafenib. Synthetic Communications. Volume 49. Issue 4. Pages 576-586. Journal; Online Computer File. (2019).
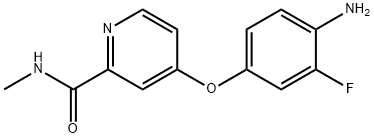
757251-39-1
246 suppliers
$11.00/250mg
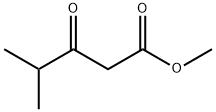
42558-54-3
385 suppliers
$10.00/5g
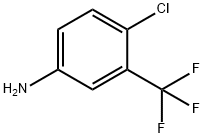
320-51-4
457 suppliers
$6.00/10g

755037-03-7
479 suppliers
$25.00/5 mg
Yield:755037-03-7 96.8%
Reaction Conditions:
with dmap in N,N-dimethyl-formamide at 140; for 4 h;Green chemistry;Temperature;
Steps:
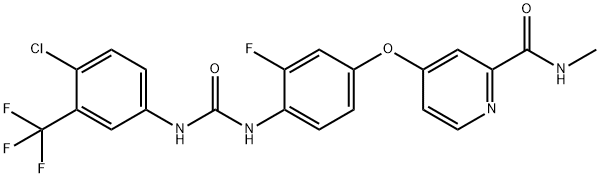
8 Preparation of regomafenib (I)
To the reaction flask, a compound IV 13.27 g, a compound V 9.93 g, a methyl isobutyryl acetate 7.32 g, a 4-dimethylaminopyridine 6.21 g, and 800 ml of DMF were sequentially added, and the mixture was stirred and dissolved, and the temperature was controlled at 140 ° C for 4 h. The TLC monitors the completion of the reaction. The reaction was stopped, the temperature was lowered to room temperature, and ethyl acetate was added for extraction. The organic layer was separated and concentrated under reduced pressure to give 25.55 g, product yield 96.8%, purity 99.93%.
References:
Shandong Luoxin Pharmaceutical Group Hengxin Pharmaceutical Co., Ltd.;Liu Zhenteng;Hou Junkai;Fan Xuezhen;Yang Taotao CN108329260, 2018, A Location in patent:Paragraph 0014; 0037; 0040; 0043-0046

757251-39-1
246 suppliers
$11.00/250mg
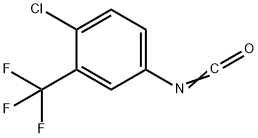
327-78-6
421 suppliers
$5.00/1g

755037-03-7
479 suppliers
$25.00/5 mg

757251-39-1
246 suppliers
$11.00/250mg
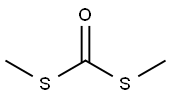
868-84-8
114 suppliers
$50.89/5g

320-51-4
457 suppliers
$6.00/10g

755037-03-7
479 suppliers
$25.00/5 mg

757251-39-1
246 suppliers
$11.00/250mg
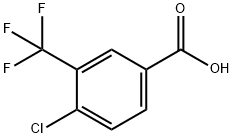
1737-36-6
134 suppliers
$15.00/1g

755037-03-7
479 suppliers
$25.00/5 mg
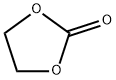
96-49-1
535 suppliers
$6.00/100g

757251-39-1
246 suppliers
$11.00/250mg

320-51-4
457 suppliers
$6.00/10g

755037-03-7
479 suppliers
$25.00/5 mg




