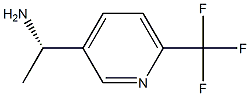
(S)-1-(6-(Trifluoromethyl)pyridin-3-yl)ethanamine synthesis
- Product Name:(S)-1-(6-(Trifluoromethyl)pyridin-3-yl)ethanamine
- CAS Number:1071435-62-5
- Molecular formula:C8H9F3N2
- Molecular Weight:190.17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1071435-62-5
20 suppliers
inquiry
Yield: 97%
Reaction Conditions:
Stage #1:(R)-2-Methyl-N-((S)-1-(6-(trifluoromethyl)pyridin-3-yl)ethyl)propane-2-sulfinamide with hydrogenchloride in 1,4-dioxane;water at 20; for 2 h;
Stage #2: with sodium hydroxide in water; pH=12
Steps:
E
E) (S)-I -(6-(trifluoromethyl)pyridin-3-yl)ethanamine.[00286] To (R)-2-Methyl-N-((S)-l -(6-(trifluoromethyl)pyridin-3-yl)ethyl)propane-2- sulfinamide (355 mg, 1.21 mmol) in a 20 mL scintilation vial was added 1 ,4-dioxane (1.6 mL) and 6.0 M of aq. HCl (0.80 mL). The reaction was stirred at rt for about 2 hours and then the dioxane was evaporated. Water (3 mL) was added and 1 M aq. NaOH was added until pH>12 was attained. The basic aqueous was extracted with CH2Cl2 (5 mL x 2). The combined organic layers were dried with Na2SO4, filtered and evaporated to obtain a clear light yellow liquid (123 mg, 54%). Chiral HPLC analysis (ChiralPac AD-H column 250 x 4.6 mm, hexane/'PrOH/Et2NH: 95/5/0.05): 97% S-isomer (9.61 min), no significant evidence of R-isomer at 10.7 min. MS: 191.2 [M+l]+;1H NMR (400 MHz, CDCl3): 8.72 (d, IH, J = 1.6 Hz), 7.92 (dd, IH, J = 8.0, 2.0 Hz), 7.66 (d, IH, J = 8.0 Hz), 4.30 (q, IH, J = 6.8 Hz), 1.55 (s, 2H), 1.43 (d, 3H, J = 6.8 Hz).
References:
RENOVIS, INC. WO2008/123963, 2008, A1 Location in patent:Page/Page column 50
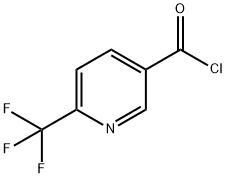
358780-13-9
97 suppliers
$45.00/100mg

1071435-62-5
20 suppliers
inquiry
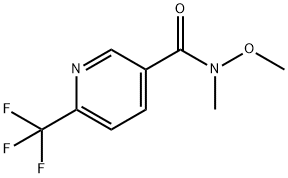
416852-53-4
10 suppliers
$450.00/1 g

1071435-62-5
20 suppliers
inquiry
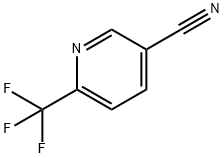
216431-85-5
207 suppliers
$8.00/1g

1071435-62-5
20 suppliers
inquiry
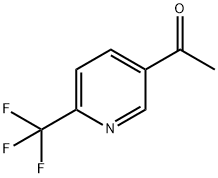
358780-14-0
127 suppliers
$32.00/100mg

1071435-62-5
20 suppliers
inquiry