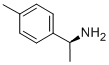
(S)-(-)-1-(P-TOLYL)ETHYLAMINE synthesis
- Product Name:(S)-(-)-1-(P-TOLYL)ETHYLAMINE
- CAS Number:27298-98-2
- Molecular formula:C9H13N
- Molecular Weight:135.21
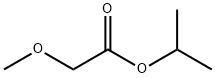
17640-21-0
38 suppliers
inquiry

42070-98-4
17 suppliers
inquiry
![Acetamide, 2-methoxy-N-[(1R)-1-(4-methylphenyl)ethyl]-](/CAS/20210305/GIF/296236-17-4.gif)
296236-17-4
0 suppliers
inquiry

27298-98-2
170 suppliers
$8.00/250mg
Yield:27298-98-2 45% ,296236-17-4 45%
Reaction Conditions:
with Novozym 435 at 23; for 3 h;Molecular sieve;Enzymatic reaction;optical yield given as %ee;enantioselective reaction;
Steps:
4.3. Preparative-scale solvent-free kinetic resolution of rac-1a-i
General procedure: One of the amines rac-1a-i (2 mmol) and isopropyl methoxyacetate (2 mmol) were added into a reaction vessel containing Novozym 435 (25 mg) and molecular sieves (4 ?, 50 mg). The reaction mixture was shaken (170 rpm) at room temperature (23 °C) if not otherwise stated. The reaction was stopped by filtering off the enzyme at (50 +/- 0.5)% conversion. Isolation of the products was performed by silica gel chromatography using a mixture of hexane and ethylacetate and/or mixture of dichloromethane and methanol as eluent.
References:
Paeivioe, Mari;Perkioe, Paeivi;Kanerva, Liisa T. [Tetrahedron Asymmetry,2012,vol. 23,# 3-4,p. 230 - 236] Location in patent:experimental part
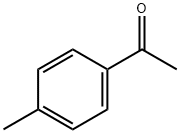
122-00-9
714 suppliers
$5.00/25g

27298-98-2
170 suppliers
$8.00/250mg
![Benzenemethanol, α-[[[[(1S)-1-(4-methylphenyl)ethyl]amino]oxy]methyl]-, (αR)-](/CAS/20210305/GIF/757195-32-7.gif)
757195-32-7
0 suppliers
inquiry
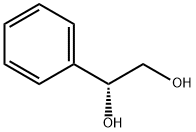
16355-00-3
228 suppliers
$13.00/1g

27298-98-2
170 suppliers
$8.00/250mg

937371-81-8
0 suppliers
inquiry

27298-98-2
170 suppliers
$8.00/250mg
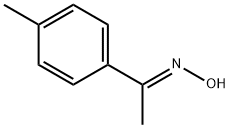
54582-23-9
6 suppliers
inquiry

27298-98-2
170 suppliers
$8.00/250mg