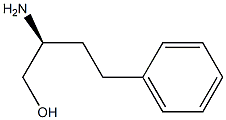
(S)-2-amino-4-phenylbutan-1-ol synthesis
- Product Name:(S)-2-amino-4-phenylbutan-1-ol
- CAS Number:27038-09-1
- Molecular formula:C10H15NO
- Molecular Weight:165.23
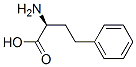
943-73-7
496 suppliers
$5.00/1g

27038-09-1
6 suppliers
inquiry
Yield:27038-09-1 97%
Reaction Conditions:
with lithium borohydride;Methyltrichlorosilane in tetrahydrofuran at 0; for 18.5 h;
Steps:
1 Reference Example 1 homo-L-phenylalaninol (Reference compound 1)
To a solution (200 mL) of lithium borohydride (3.6 g, 170 mmol) in tetrahydrofuran was added trichloromethylsilane (36 g, 340 mmol) under ice-cooling, and the mixture was stirred for 30 min. To this solution was slowly added homo-L-phenylalanine (10 g, 56 mmol) under the same conditions and the mixture was stirred for 18 hr. To this solution was added methanol until generation of a hydrogen gas stopped. After concentration under reduced pressure, 5% aqueous sodium hydroxide solution was added and the mixture was extracted twice with dichloromethane. The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to give Reference compound 1 (9.2 g, 97%) as a white solid. mp 40.3-41.3°C. 1H-NMR (300 MHz, DMSO-d6)δ:1.39 (m, 1H), 1.64 (m, 1H), 2.54-2.75 (m, 3H), 3.17 (dd, 1H, J = 7.7, 4.1 Hz), 3.26 (m, 3H), 3.29 (dd, 1H, J = 10.5, 4.8 Hz), 7.13-7.30 (m, 5H).
References:
EP1491537,2004,A1 Location in patent:Page 13
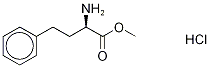
60425-49-2
42 suppliers
$165.00/1g

27038-09-1
6 suppliers
inquiry
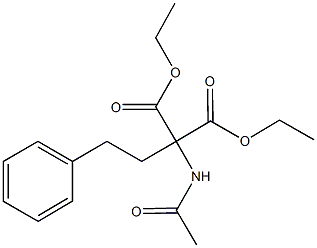
5463-92-3
44 suppliers
$215.25/250mg

27038-09-1
6 suppliers
inquiry
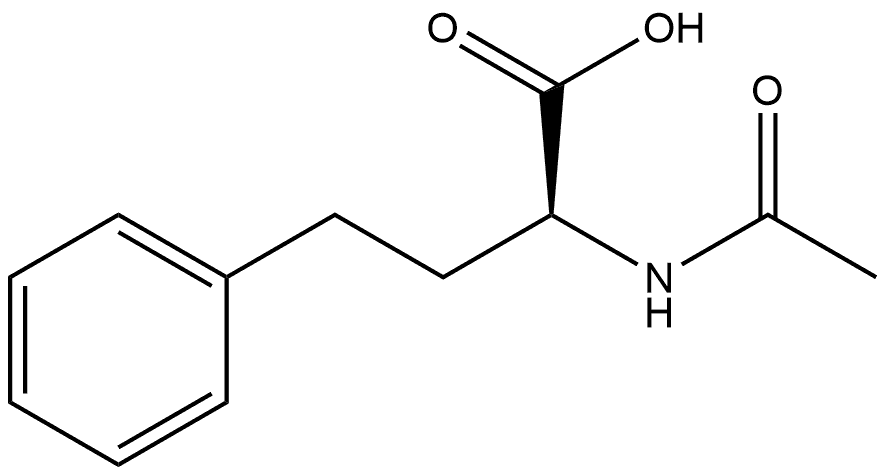
96613-91-1
0 suppliers
inquiry

27038-09-1
6 suppliers
inquiry
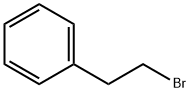
103-63-9
504 suppliers
$9.00/10g

27038-09-1
6 suppliers
inquiry