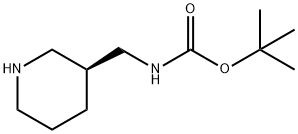
(S)-Tert-butyl (piperidin-3-ylmethyl)carbamate synthesis
- Product Name:(S)-Tert-butyl (piperidin-3-ylmethyl)carbamate
- CAS Number:1016167-99-9
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
142643-29-6

1016167-99-9
The general procedure for the synthesis of tert-butyl (S)-(piperidin-3-ylmethyl)carbamate from 3-Boc-aminomethylpiperidine is as follows:Example 4 Synthesis of (3S)-N-(tert-butoxycarbonyl)-3-aminomethylpiperidine (Compound 5): N-(tert-butoxycarbonyl)-3-aminomethylpiperidine (10 g, 47 mmol, 1 equiv), (-)-O,O' -di-β-tosyl-L-tartaric acid (15.52 g, 47 mmol, 1 eq.) were mixed with dry methanol (100 ml) and slowly heated to reflux to form a homogeneous solution. The reaction mixture was cooled to room temperature and stirred for 5-6 hours. The resulting white solid was filtered and washed with a minimal amount of anhydrous methanol. The crude product was purified by recrystallization from methanol. The resulting compound was suspended in distilled water (25 ml) and cooled to 0°C. 10% sodium carbonate solution (100 ml) was added in batches until the reaction mixture was basic and stirring was continued for 10 minutes. The reaction mixture was extracted with ethyl acetate (5 x 50 ml), the organic layer was separated and concentrated under reduced pressure after drying to give the target compound 3. Yield: 3.28 g (65%). [α]D: +11.03 (c=0.10, methanol). Melting point: 64-66°C. IR (pure): 3360, 2972, 1703, 1519, 1455, 1365, 1255, 1172 cm-1. 1H NMR (CDCl3, 300MHz) δ: 1.01-1.21 (m, 2H, H-4); 1.39 (s, 9H, -OC(CH3)3); 1.57- 1.72 (m, 3H, H-3, H-5); 2.20-2.31 (m, 1H, CHaNHBoc); 2.49-2.56 (m, 1H, CHbNHBoc); 2.90-3.03 (m, 4H, H-2, H-4); 4.77 (brs, 1H, NH).13C NMR (CDCl3, 300MHz) δ : 26.56, 29.03, 29.58, 38.44, 44.99, 47.46, 51.16, 79.73, 156.72. FAB MS (m/z): 215 (M+1, 114).

142643-29-6
159 suppliers
$6.00/100mg

1016167-99-9
68 suppliers
inquiry
Yield: 65%
Reaction Conditions:
Stage #1:tert-butyl (piperidin-3-ylmethyl)carbamate with (-)-di-p-toluoyl-L-tartaric acid in methanolReflux;Resolution of racemate;
Stage #2: with sodium carbonate in water at 0;Resolution of racemate;
Steps:
4
Example 4Synthesis of (3S)-N-(t-butoxycarbonyl)-3-aminomethyl-piperidine (compound 5); N-(t-butoxycarbonyl)-3-aminomethyl piperidine (10 g, 1eq, 47 mmol), (-)-O, O -Di-p-tolouyl- L-tartaric acid (15.52, 1eq, 47 mmol) and dry methanol (100 ml) were mixed and heated slowly to refluxing to just to get a uniform solution. The reaction mixture was cooled to room temperature and stirred at this temperature for 5-6 hours. White solid formed was filtered out and washed with minimum quantity of dry methanol. The crude solid was recrystallized from methanol. The compound was suspended in distilled water (25 ml) and cooed to 0 °C. 10% solution of sodium carbonate solution (100 ml) was then added portion wise, until basic, with stirring for 10 min. The reaction mixture was extracted with ethyl acetate (5 x 50 ml). The organic layer was separated, dried and concentrated under reduced pressure to obtain the compound of formula 3.Yield: 3.28g, 65 %L J D : + 11.03 (c = 0.10; Methanol)MP: 64-66 °CIR (Neat):3360, 2972, 1703, 1519, 1455, 1365, 1255, 1172 cm"1 H NMR (CDCI3, 300 MHz)δ 1.01 -1.21 (m, 2H, H-4); 1.39 (s, 9H, -0-C(CH3)3); 1.57-1.72 (m, 3H, H-3, H-5); 2.20-2.31 (m, 1 H, CHaNHBoc); 2.49-2.56 (m, 1 H, CHbNHBoc); 2.90-3.03 (m, 4H, H-2, H-4); 4.77(brs, 1 H, NH)13C NMR(CDCI3, 300 MHz)26.56, 29.03, 29.58, 38.44, 44.99, 47.46, 51.16, 79.73, 156.72FAB MS (m/z):215(M+1 f , 1 14
References:
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH;DIKSHIT, Dinesh, Kumar;DIKSHIT, Madhu;SIDDIQUI, Tanveer, Irshad;KUMAR, Anil;BHATTA, Rabi, Sankar;JAIN, Girish, Kumar;BARTHWAL, Manoj, Kumar;MISRA, Ankita;KHANNA, Vivek;PRAKASH, Prem;JAIN, Manish;SINGH, Vishal;GUPTA, Varsha;DWIVEDI, Anil, Kumar WO2012/104866, 2012, A1 Location in patent:Page/Page column 17-18

1026661-43-7
35 suppliers
$45.00/10mg

1016167-99-9
68 suppliers
inquiry