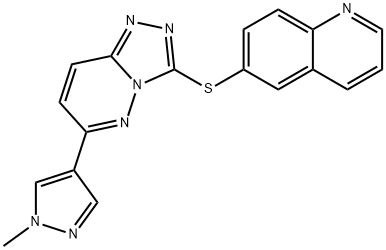
SGX-523 synthesis
- Product Name:SGX-523
- CAS Number:1022150-57-7
- Molecular formula:C18H13N7S
- Molecular Weight:359.41
5332-25-2
![1,2,4-Triazolo[4,3-b]pyridazine-3(2H)-thione, 6-(1-methyl-1H-pyrazol-4-yl)-](/CAS/20210305/GIF/1022151-55-8.gif)
1022151-55-8

1022150-57-7
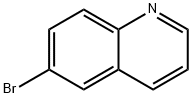
Method b: 6-bromoquinoline (550 mg, 2.64 mmol) and diisopropylethylamine (1.13 mL, 6.46 mmol) were dissolved in N,N-dimethylacetamide (DMA, 4 mL) under nitrogen protection and degassed by nitrogen bubbling for 20 min. Subsequently, tris(dibenzylideneacetone)dipalladium (105 mg, 0.108 mmol, Strem catalyst), Xantphos (125 mg, 0.216 mmol), and 6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazine-3-thiol (500 mg, 2.155 mmol ). The reaction mixture was stirred at 100 °C for 22 h until the solid was completely dissolved. After completion of the reaction, the mixture was cooled to room temperature and filtered through a short column of silica gel, using N,N-dimethylformamide (DMF) as eluent. The filtrate was poured directly into an ice/water mixture and allowed to stand for 15 minutes to precipitate the product. The precipitate was collected by filtration and washed with water. The resulting solid was ground with ether, filtered and dried under vacuum to give 770 mg of yellow crude product. The crude product was stirred in refluxing isopropanol for 1 h. The product was hot filtered, washed with isopropanol, and dried in a vacuum oven at 70 °C for 3 days to give a final 500 mg of 6-((6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)sulfanyl)quinoline as a yellow solid with 8% impurities (64% yield).

5332-25-2
398 suppliers
$6.00/5g
![1,2,4-Triazolo[4,3-b]pyridazine-3(2H)-thione, 6-(1-methyl-1H-pyrazol-4-yl)-](/CAS/20210305/GIF/1022151-55-8.gif)
1022151-55-8
1 suppliers
inquiry

1022150-57-7
154 suppliers
$39.00/1mg
Yield:1022150-57-7 64%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;tris-(dibenzylideneacetone)dipalladium(0);4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in N,N-dimethyl acetamide at 100; for 22 h;Product distribution / selectivity;
Steps:
4.b
Method b; [0358] A solution of 6-bromoquinoline (550 mg, 2.64 mmol), diisopropylethylamine (1.13 niL, 6.46 mmol) in DMA (4 mL) under nitrogen was degassed by bubbling in nitrogen for 20 min. Tris(dibenzylideneacetone)dipalladium (105 mg, 0.108 mmol, Strem catalyst), Xantphos (125 mg, 0.216 mmol), and 6-(l-methyl-lH-pyrazol-4-yl)-[l,2,4]triazolo[4,3- ]pyridazine-3 -thiol (500 mg, 2.155 mmol) were added under a stream of nitrogen. The reaction mixture was stirred at 1000C for 22h, with disappearance of the solid suspension. The reaction mixture was cooled to room temperature and filtered through a plug of silica gel, using DMF as eluent. The organics were then directly poured onto ice/water mixture and left standing for 15 min. The precipitate was filtered and washed with water. The resulting cake was grinded in diethyl ether, filtered, and dried in vacuo to yield 770 mg of a yellow solid. The solid was stirred in refluxing isopropanol for Ih, filtered, washed with isopropanol, and dried in a vacuum oven at 7O0C for 3 days to provide 500 mg of 6-[6-(l- methyl-lH-pyrazol-4-yl)-[l,2,4]triazolo[4,3-6]pyridazin-3-ylsulfanyl]-quinoline as a yellow solid with 8% impurity (64% yield).
References:
WO2008/51808,2008,A2 Location in patent:Page/Page column 101