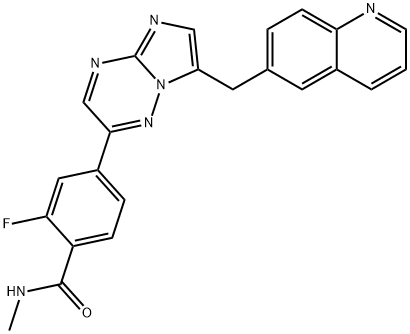
Capmatinib synthesis
- Product Name:Capmatinib
- CAS Number:1029712-80-8
- Molecular formula:C23H17FN6O
- Molecular Weight:412.42
![2-fluoro-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzoicacid](/CAS/20180702/GIF/1029714-88-2.gif)
1029714-88-2

74-89-5

1029712-80-8
Example 20 Synthesis of 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide (15): 2-fluoro-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzoic acid (14, 331.4 g, 0.914 mol, 1.0 eq.) and (benzotriazol-1-yloxy)tripyrrolidinylphosphonium hexafluorophosphate (PyBOP, 570 g, 1.1 mol, 1.2 eq.) were dissolved in N,N-dimethylformamide (DMF, 3700 mL). To this solution, a tetrahydrofuran solution of 2M methylamine (1830 mL, 3.656 mol, 4.0 eq.) was slowly added for 15 min at room temperature. The reaction temperature was raised to 30 °C during the addition and the reaction mixture became homogeneous. Subsequently, triethylamine (TEA, 382 mL, 2.742 mol, 3.0 eq.) was added to the reaction mixture and the reaction mixture was stirred for 2-4 h at room temperature. The progress of the reaction was monitored by LC/MS and after confirming the completion of the coupling reaction, water (950 mL) was added to the reaction mixture. The resulting suspension was cooled to 0-5 °C in an ice bath and stirred at this temperature for 30 min. The solid was collected by filtration and washed with water (200 mL). The wet solid filter cake was suspended in a solvent mixture of water and acetonitrile (1:1 v/v, 2000 mL) and stirred for 1 hour at room temperature. The solid was collected by filtration, washed sequentially with water and acetonitrile, and then dried to constant weight in a vacuum oven at 40-45 °C to afford 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide (15, 322 g, 85.4% yield) as a yellow to bright yellow powder.
![Benzamide, 2-fluoro-4-[7-(6-quinolinylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]-](/CAS/20210305/GIF/1029713-99-2.gif)
1029713-99-2
5 suppliers
inquiry

77-78-1
311 suppliers
$22.00/25g

1029712-80-8
262 suppliers
$50.00/20mg
Yield:1029712-80-8 90%
Reaction Conditions:
Stage #1: 2-fluoro-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamidewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 5; for 0.5 h;
Stage #2: dimethyl sulfate in N,N-dimethyl-formamide;mineral oil at 60; for 2 h;
Steps:
1.2 Step 2: Preparation of camatinib
Add DMF 500ml, 20.0g (0.05mol) of formula II compound to a 2L reaction flask, stir to dissolve, cool to 0-5°C, add 2.4g (0.06mol) 60% sodium hydride in mineral oil in batches, and complete the addition, Stirring for 0.5 hours, adding 7.5 g (0.06 mol) of dimethyl sulfate dropwise, after dropping, slowly increasing to 60° C. and reacting for 2 hours. After the reaction is complete, add dilute hydrochloric acid dropwise to quench, concentrate under reduced pressure to remove part of the solvent, pour into water, extract with ethyl acetate, adjust the aqueous phase to alkaline, extract with ethyl acetate, wash with water, concentrate the organic phase to obtain 18.6 g of yellow solid, it is the title compound, the yield is 90%, and the HPLC purity is 99.5%.
References:
CN111825678,2020,A Location in patent:Paragraph 0034-0035; 0038-0039
![2-fluoro-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzoicacid](/CAS/20180702/GIF/1029714-88-2.gif)
1029714-88-2
14 suppliers
inquiry

74-89-5
0 suppliers
$13.44/25ML

1029712-80-8
262 suppliers
$50.00/20mg
![2-fluoro-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzonitrile](/CAS/20180703/GIF/1029714-87-1.gif)
1029714-87-1
14 suppliers
inquiry

1029712-80-8
262 suppliers
$50.00/20mg
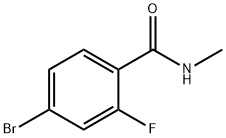
749927-69-3
314 suppliers
$40.00/1g

1029712-80-8
262 suppliers
$50.00/20mg
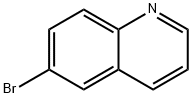
5332-25-2
396 suppliers
$6.00/5g

1029712-80-8
262 suppliers
$50.00/20mg