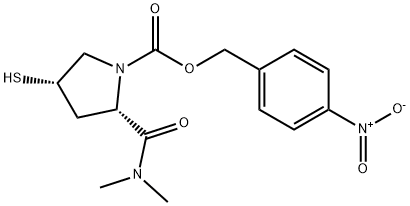
Side chain for meropenem synthesis
- Product Name:Side chain for meropenem
- CAS Number:96034-64-9
- Molecular formula:C15H19N3O5S
- Molecular Weight:353.39
![1,2-Pyrrolidinedicarboxylic acid, 4-mercapto-, 1-[(4-nitrophenyl)methyl] ester, (2S,4S)-](/CAS/20200611/GIF/127966-47-6.gif)
127966-47-6
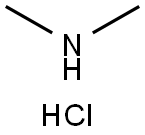
506-59-2

96034-64-9
600 mL of N,N-dimethylformamide (DMF) was added to the reaction flask and the temperature of the reaction system was lowered to 0°C to 5°C. Subsequently, 179.5 g (0.55 mol) of (2S,4S)-1-carboxy-4-mercaptopyrrolidine (Formula VI) was added. Next, 66.8 g (0.66 mol) of diisopropylethylamine was added and the reaction mixture was stirred for 10 minutes. Then, 89.7 g (1.10 mol) of dimethylamine hydrochloride was added, and the reaction temperature was maintained between 0 °C and 5 °C, and the reaction continued to be stirred for 3 hours. Upon completion of the reaction, 500 mL of ice water was quickly added to the reaction mixture and stirred to precipitate a large amount of solid. The solid product was collected by filtration and the filter cake was washed with 200 mL of water. Finally, the product was dried under reduced pressure at 65 °C for 15 h to afford 179.4 g (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-(N,N-dimethylcarbamoyl)-4-mercapto-pyrrolidine (Formula VII) in 92.3% molar yield and 99.3% HPLC purity. The mass spectral data of compound VII were as follows: molecular formula C15H19N3O5S, molecular weight 353.4, [M+H]+: 354.6.
![1,2-Pyrrolidinedicarboxylic acid, 4-mercapto-, 1-[(4-nitrophenyl)methyl] ester, (2S,4S)-](/CAS/20200611/GIF/127966-47-6.gif)
127966-47-6
0 suppliers
inquiry

506-59-2
558 suppliers
$5.00/5G

96034-64-9
331 suppliers
$8.00/250mg
Yield:96034-64-9 92.3%
Reaction Conditions:
Stage #1: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-carboxy-4-mercaptopyrrolidinewith N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 0 - 5; for 0.166667 h;
Stage #2: N,N-dimethylammonium chloride in N,N-dimethyl-formamide at 0 - 5; for 3 h;Concentration;
Steps:
10 Preparation of (2S, 4S) l-p-nitrobenzyloxycarbonyl-2- (N, N-dimethylcarbamoyl) -4-mercaptopyrrolidine (Formula VII)
The reaction flask was charged with 600 mL of DMF, and 179.5 g (0.55 mol) of (2S, 4S) -1- Carboxy-4-mercaptopyrrolidine (Formula VI) was prepared by reducing the temperature to 0 ° C to 5 ° C, adding 66.8 g (0.66 mol) Diisopropylethylamine, After stirring for 10 minutes, 89.7 g (1.10 mol) of dimethylamine hydrochloride was added and the mixture was stirred at 0 ° C to 5 ° C After 3 hours of reaction, 500 mL of purified ice water was quickly added, and a large amount of solid was precipitated under stirring. After filtration, the cake was washed with 200 mL of water , And dried under reduced pressure at 65 ° C for 15 hours to obtain 179.4 g of (2S, 4S) -1-p-nitrobenzyloxycarbonyl-2- (N, N-dimethylcarbamoyl Yl) -4-mercaptopyrrolidine (Formula VII), 92.3% molar yield, HPLC purity 99.3%. Compound VII mass spectrometry data are as follows: C15H19N3O5S, molecular weight: 353.4, [M + H] & lt; + & gt ;: 354.6.
References:
CN105439932,2016,A Location in patent:Paragraph 0075; 0076; 0077
![1-Pyrrolidinecarboxylic acid, 3,3'-dithiobis[5-[(dimethylamino)carbonyl]-, 1,1'-bis[(4-nitrophenyl)methyl] ester, (3S,3'S,5S,5'S)-](/CAS/20211123/GIF/936226-36-7.gif)
936226-36-7
6 suppliers
inquiry

96034-64-9
331 suppliers
$8.00/250mg
![1-Pyrrolidinecarboxylic acid, 4-(acetylthio)-2-[(dimethylamino)carbonyl]-, (4-nitrophenyl)methyl ester, (2S,4S)-](/CAS/20200331/GIF/96034-61-6.gif)
96034-61-6
0 suppliers
inquiry

96034-64-9
331 suppliers
$8.00/250mg

506-59-2
558 suppliers
$5.00/5G
![2-Thia-5-azabicyclo[2.2.1]heptane-5-carboxylic acid, 3-oxo-, (4-nitrophenyl)methyl ester, (1S,4S)-](/CAS/20200611/GIF/151072-00-3.gif)
151072-00-3
26 suppliers
inquiry

96034-64-9
331 suppliers
$8.00/250mg
51-35-4
884 suppliers
$6.00/5g

96034-64-9
331 suppliers
$8.00/250mg