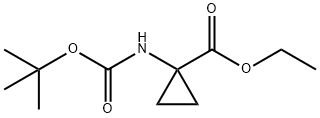
SINOVA SL-03967 synthesis
- Product Name:SINOVA SL-03967
- CAS Number:107259-05-2
- Molecular formula:C11H19NO4
- Molecular Weight:229.27

24424-99-5
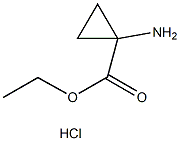
42303-42-4

107259-05-2
The general procedure for the synthesis of ethyl 1-(BOC-amino)cyclopropane-1-carboxylate from di-tert-butyl dicarbonate and ethyl 1-aminocyclopropane-1-carboxylate hydrochloride was as follows: to a solution of 1-aminocyclopropane carboxylic acid ethyl ester hydrochloride (3.22 g, 19.4 mmol) in dichloromethane (35 mL) was added triethylamine (2.71 mL, 19.4 mmol) to form a suspension. A solution of dichloromethane (5 mL) of di-tert-butyl dicarbonate (4.24 g, 19.4 mmol) was added slowly and dropwise over about 5 hours. The reaction mixture was stirred overnight at room temperature. After completion of the reaction, extraction was carried out by adding 1 M aqueous KHSO4 solution (100 mL) and dichloromethane (50 mL). The organic layer was separated, dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure and co-evaporated with tetrahydrofuran (2×) to give 4.31 g (97% yield) of ethyl 1-(BOC-amino)cyclopropanecarboxylate.

24424-99-5
871 suppliers
$13.50/25G

42303-42-4
166 suppliers
$6.00/1g

107259-05-2
59 suppliers
$60.00/100mg
Yield: 97%
Reaction Conditions:
with triethylamine in dichloromethane at 23;
Steps:
1.3.3.1 Step 1: Eth vi 1 -((tert-butoxycarbonyl )am ino)cyclopropanecarboxylate
To a solution of ethyl 1-aminocyclopropanecarboxylate hydrochloride (3.22 g, 19.4 mmol) in DCM (35 mL) was added triethylamine (2.71 mL, 19.4 mmol) upon which a suspension was obtained. A solution of Boc2O (4.24 g, 19.4 mmol) in DCM (5 mL) was added dropwise over ca. 2 mm. The reaction mixturewas stirred at RT overnight. Aqueous I M KHSO4 (100 mL) and DCM (50 mL) were added. The organic layer was separated, dried (Na2SO4), evaporated under reduced pressure and co-evaporated with THF (2x), to afford 4.31 g (97%) of the desired product.
References:
GRÜNENTHAL GMBH;REICH, Melanie;SCHUNK, Stefan;JAKOB, Florian;DAMANN, Nils;HAURAND, Michael;HAMLYN, Richard;ROGERS, Marc;SUTTON, Kathy;SKONE, Philip WO2015/90603, 2015, A1 Location in patent:Page/Page column 59; 60
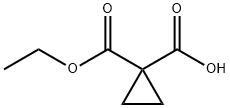
3697-66-3
120 suppliers
$16.00/250mg

75-65-0
784 suppliers
$10.00/10ml

107259-05-2
59 suppliers
$60.00/100mg

24424-99-5
871 suppliers
$13.50/25G
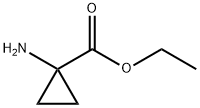
72784-47-5
52 suppliers
$45.00/10mg

107259-05-2
59 suppliers
$60.00/100mg
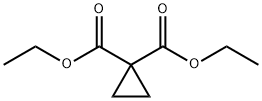
1559-02-0
323 suppliers
$5.00/5g

107259-05-2
59 suppliers
$60.00/100mg