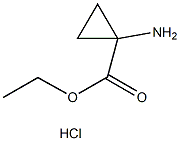
Ethyl 1-aminocyclopropanecarboxylate hydrochloride synthesis
- Product Name:Ethyl 1-aminocyclopropanecarboxylate hydrochloride
- CAS Number:42303-42-4
- Molecular formula:C6H12ClNO2
- Molecular Weight:165.62

64-17-5
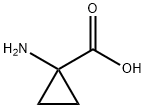
22059-21-8

42303-42-4
Thionyl chloride (150 mL, 2.056 mol) was slowly added to a suspension of 1-aminocyclopropanecarboxylic acid (100 g, 0.989 mol) in anhydrous ethanol (1 L) below 0 °C. The reaction mixture was stirred at 70 °C for 20 hours. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent: methanol, Rf = 0.4) to confirm that most of the raw materials had been consumed. Upon completion of the reaction, the solution was concentrated to give 210 g of crude product. The residue was dissolved in water and the pH was adjusted to 9-10 with potassium carbonate.Subsequently, the aqueous layer was extracted with dichloromethane (1L x 3). The organic layers were combined and concentrated to dryness. The residue was dissolved in ethyl acetate (300 mL) and a hydrochloride solution of ethyl hydrochloride (250 mL, 4 M) was slowly added at below -30°C. The mixture was stirred at 0 °C for 30 min, during which time a solid precipitated. Filtration under nitrogen protection gave ethyl 1-aminocyclopropane-1-carboxylate hydrochloride (132 g, 80.6% yield) as a white solid. The 1H-NMR data of the free amine were as follows (400 MHz, chloroform-d): δ[ppm] = 0.91-1.02 (m, 2H), 1.15-1.30 (m, 5H), 2.17 (s, 2H), 4.10 (d, 2H).

64-17-5
825 suppliers
$10.00/50g

22059-21-8
467 suppliers
$14.00/1g

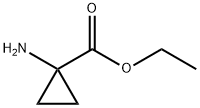
42303-42-4
166 suppliers
$6.00/1g
Yield:42303-42-4 100%
Reaction Conditions:
with thionyl chloride at 0; for 2 h;Reflux;Green chemistry;
Steps:
3 Preparation of compound of formula III
The compound of the formula II (500.0 g, 4.95 mol) was added to ethanol (10 L) at room temperature, and thionyl chloride(883.4g, 7.43 mol) was added dropwise at 0 °C, and the reaction mixture was heated to reflux and stirred for 2 h. The solution was cooled to room temperature, concentrated, and washed three times with THF (1L×3) to give the compound of formula III, 819.8 g, yield 100%.
References:
Nanjing Yaoshi Technology Co., Ltd.;Nanjing Furunkaide Bio-pharmaceutical Co., Ltd.;Shu Qingning;Wang Wei CN108863958, 2018, A Location in patent:Paragraph 0073; 0074; 0075; 0076
107259-05-2
59 suppliers
$60.00/100mg

42303-42-4
166 suppliers
$6.00/1g
72784-47-5
52 suppliers
$45.00/10mg

42303-42-4
166 suppliers
$6.00/1g
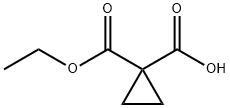
3697-66-3
119 suppliers
$16.00/250mg

42303-42-4
166 suppliers
$6.00/1g
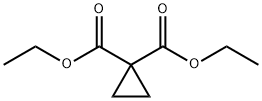
1559-02-0
323 suppliers
$5.00/5g

42303-42-4
166 suppliers
$6.00/1g