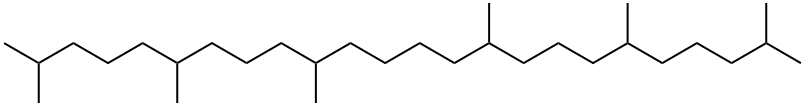
Squalane synthesis
- Product Name:Squalane
- CAS Number:111-01-3
- Molecular formula:C30H62
- Molecular Weight:422.81
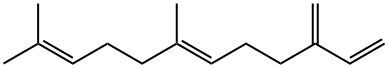
18794-84-8
142 suppliers
$28.00/1G
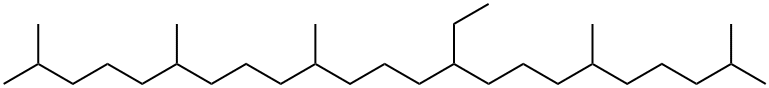
1350472-07-9
0 suppliers
inquiry

111-01-3
432 suppliers
$12.00/25g
Yield:111-01-3 6.2 %Chromat. ,1350472-07-9 59.5 %Chromat.
Reaction Conditions:
Stage #1: (E)-β-farnesenewith diethylaluminium chloride in n-heptane;toluene; for 12 h;Glovebox;Schlenk technique;Inert atmosphere;Heating;
Stage #2: with palladium 10% on activated carbon;hydrogen in toluene at 85; under 30003 Torr; for 12 h;Autoclave;Reagent/catalyst;
Steps:
3c; 5 Example 3c: Homogeneous catalysis with Cp-arene TiCl3
Example 5: Hydrogenation of the crude dimerization reaction obtained in examples 3 and 4
General procedure: In a glove box, the catalyst Cp-arene TiCh (0.016 mmol, 6 mg, 1 eq) and toluene (3 mL) were charged in a 20 mL schlenk. Then, to this mixture was added a solution of Et2AlCl (1 M in heptane, 0.3 mL, 18 eq) and stirred for 20 min. After that, beta- farnesene (8 mmol, 1.6 g, S/C = 500) was added to this mixture and stirred for 12 h at 110°C. Then, the mixture was cooled, removed from the glovebox and under argon, 1.5 mL of isopropanol was added to quench the reaction. An aliquot of the crude is injected in GC-FID to determine if there is any conversion. Finally, the crude of the dimerization reaction was filtrated through a small neutral alumina path on Buchner fritted disc (N°4) funnel, washed several times with toluene. The solvent was evaporated in the rotavapor and the product was stored in the fridge.
The crude of the dimerization reaction (0.089 g) was charged in a stainless steel autoclave with 10 wt% Pd/C (150 mg), 5 mL of toluene, 40 bar of H2 and stirred for 12 h at 85 °C. After that, an internal standard nonadecane (80 mg) was added to the hydrogenated mixture and an aliquot was injected in the GC-FID to obtain the conversion and selectivity on squalane and isosqualane. The conversion was mainly calculated based on the latter method unless further specification. The diene conversion refers to the amount in percentage by weight of diene that has reacted.
References:
WO2018/11156,2018,A1 Location in patent:Page/Page column 24; 26; 28
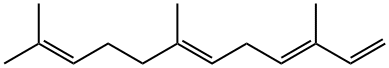
502-61-4
88 suppliers
inquiry

111-01-3
432 suppliers
$12.00/25g
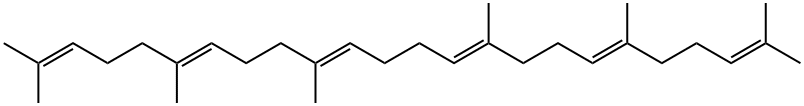
111-02-4
265 suppliers
$15.68/25ML

111-01-3
432 suppliers
$12.00/25g
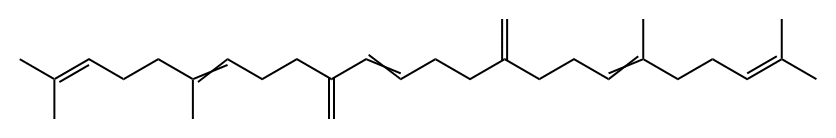
51627-57-7
0 suppliers
inquiry

111-01-3
432 suppliers
$12.00/25g

111-02-4
265 suppliers
$15.68/25ML

111-01-3
432 suppliers
$12.00/25g