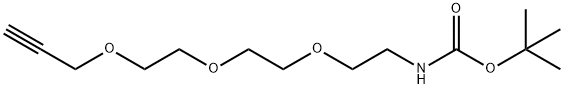
t-Boc-N-Amido-PEG3-propargyl synthesis
- Product Name:t-Boc-N-Amido-PEG3-propargyl
- CAS Number:1333880-60-6
- Molecular formula:C14H25NO5
- Molecular Weight:287.35
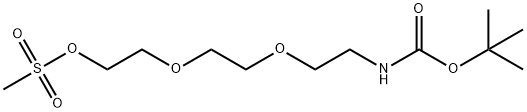
![(2-[2-(2-HYDROXY-ETHOXY)-ETHOXY]-ETHYL)-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/139115-92-7.gif)
139115-92-7
139 suppliers
$23.00/100mg
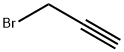
106-96-7
339 suppliers
$30.00/25g

1333880-60-6
46 suppliers
inquiry
Yield:1333880-60-6 72%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium iodide;potassium hydroxide in tetrahydrofuran at 25; for 16 h;Inert atmosphere;
Steps:
Tert-butyl N-[2-[2-(2-prop-2-ynoxyethoxy)ethoxy]ethyl]carbamate (Intermediate VS)
A mixture of tert-butyl N-[2-[2-(2-hydroxyethoxy)ethoxy]ethyl]carbamate (3.00 g, 12.0 mmol), 3-bromoprop-1-yne (1.72 g, 14.4 mmol, 1.24 mL), TBAI (356 mg, 962 umol), KI (299 mg, 1.81 mmol) and KOH (675 mg, 12.0 mmol) in THF (30 mL) was degassed and purged with N2 for 3 times, and then the mixture was stirred at 25° C. for 16 hrs under N2 atmosphere. On completion, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel chromatography to give the title compound (2.50 g, 72% yield) as light yellow oil. 1H NMR (400 MHz, CDCl3) δ 5.05 (s, 1H), 4.22 (d, J=2.4 Hz, 2H), 3.76-3.60 (m, 8H), 3.55 (t, J=5.2 Hz, 2H), 3.35-3.27 (m, 2H), 2.44 (t, J=2.4 Hz, 1H), 1.47 (s, 9H).
References:
Kymera Therapeutics, Inc.;Mainolfi, Nello;Ji, Nan;Kluge, Arthur F.;Weiss, Matthew M.;Zhang, Yi US2019/192668, 2019, A1 Location in patent:Paragraph 4042; 4043
![(2-[2-(2-HYDROXY-ETHOXY)-ETHOXY]-ETHYL)-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/139115-92-7.gif)
139115-92-7
139 suppliers
$23.00/100mg

1333880-60-6
46 suppliers
inquiry
![2-[2-(2-AMINOETHOXY)ETHOXY]ETHANOL](/CAS/GIF/6338-55-2.gif)
6338-55-2
228 suppliers
$10.00/100mg

1333880-60-6
46 suppliers
inquiry

24424-99-5
861 suppliers
$13.50/25G

1333880-60-6
46 suppliers
inquiry