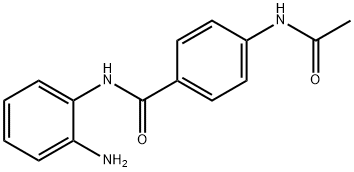
Tacedinaline synthesis
- Product Name:Tacedinaline
- CAS Number:112522-64-2
- Molecular formula:C15H15N3O2
- Molecular Weight:269.3
![Carbamic acid, N-[2-[[4-(acetylamino)benzoyl]amino]phenyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1299346-13-6.gif)
1299346-13-6

112522-64-2
GENERAL STEPS: Trifluoroacetic acid (22 mL) was added slowly and dropwise to a solution of tert-butyl (2-(4-acetylaminobenzoyl)phenyl)carbamate 1.5 (3.5 g, 9.5 mmol) in anhydrous dichloromethane (55 mL) at 0 °C. The reaction mixture was slowly warmed to 23 °C and stirred at this temperature for 2 h until the reaction was complete. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was diluted with water and the pH was adjusted with saturated aqueous sodium bicarbonate to about 8. The precipitate precipitated was filtered, washed sequentially with water and ether, and finally dried under vacuum to afford 4-acetamido-N-(2-aminophenyl)benzamide 1.6 as an off-white solid in a yield of 2.4 g (96% yield).1H NMR (500 MHz, d6-DMSO) δ 10.18 (s, 1H), 9.54 (s, 1H), 7.93 (d, J = 8.5Hz, 2H), 7.69 (d, J = 8.5Hz, 2H), 7.15 (d, J = 7.5Hz, 1H), 6.96 (t, J = 7.5Hz, 1H), 6.78 (d, J = 7.5Hz, 1H), 6.59 (t, J = 7.5Hz, 1H ), 4.88 (s, 2H), 2.08 (s, 3H).MS (ESI+): m/z 269.9 [M + H]+.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112522-64-2
201 suppliers
$17.00/10mg
Yield: 92%
Reaction Conditions:
with sodium hydrogencarbonate in ethanol;water; pH=8 for 1.5 h;
Steps:
15
The dried solid of 4-acetamido-N-(2-aminophenyl)benzamide trifluoroacetate salt was suspended in a solution of 1 : 1 EtOH:H20 (320mL). Saturated NaHC03 solution (lOOmL) was added slowly to adjust the pH to 8. The resultant slurry was stirred for 1.5 hours. The precipitates were filtered and washed with water (2 X 60mL). After drying at 40°C in vacuo for 16h, 4-acetamido-N-(2-aminophenyl)benzamide (21.3g, 92% yield, 97.0% HPLC purity) was isolated as crystalline Form B (white solid).1HNMR (400 Hz, d6-DMSO) δ: 10.22 (s, 1H), 9.58 (s, 1H), 7.94 (d, J= 8.8 Hz, 2H), 7.70 (d, J = 8.8 Hz, 2H), 7.16 (dd, J = 1.2 Hz, 8.0 Hz, 1H), 6.97 (app dt, J= 1.6 Hz, 8.4 Hz, 1H), 6.79 (dd, J = 1.2 Hz, 8.0 Hz, 1H), 6.6 ( app dt, J= 1.6 Hz, 8.4 Hz, 2H), 4.90 (s, 2H), 2.10 (s, 3H).
References:
THE BROAD INSTITUTE, INC.;HOLSON, Edward;WAGNER, Florence;STAHLY, G., Patrick WO2012/3413, 2012, A1 Location in patent:Page/Page column 52-53
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112522-64-2
201 suppliers
$17.00/10mg
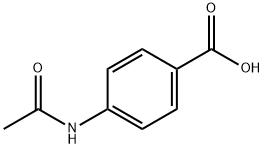
556-08-1
350 suppliers
$6.00/10g
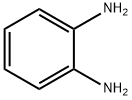
95-54-5
536 suppliers
$9.00/1g

112522-64-2
201 suppliers
$17.00/10mg
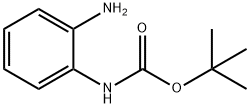
146651-75-4
145 suppliers
$6.00/250mg

112522-64-2
201 suppliers
$17.00/10mg

95-54-5
536 suppliers
$9.00/1g

112522-64-2
201 suppliers
$17.00/10mg