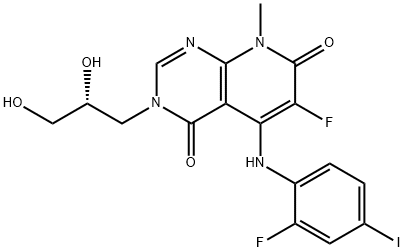
TAK-733 synthesis
- Product Name:TAK-733
- CAS Number:1035555-63-5
- Molecular formula:C17H15F2IN4O4
- Molecular Weight:504.23
![(R)-3-(2,3-DIHYDROXYPROPYL)-5-(2-FLUORO-4-IODOPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180601/GIF/1035555-51-1.gif)
1035555-51-1

1035555-63-5
Example 18: (i) To a solution of 3-(2,3-dihydroxypropyl)-5-(2-fluoro-4-iodophenylamino)-8-methylpyrido[2,3-d]pyrimidin-4,7(3H,8H)-dione (Example 6) (1 g, 2.06 mmol, 1 eq.) in DMF (19 mL) was added dropwise under nitrogen protection at ambient temperature a solution of Selectfluor (801 mg, 2.26 mmol, 1.1 eq.) in a mixture of acetonitrile (9 mL) and DMF (5 mL). The reaction mixture was stirred at ambient temperature for 10 minutes and then filtered. The filtrate was purified by preparative LC/MS (30-55% CH3CN/H2O) to afford (R)-3-(2,3-dihydroxypropyl)-6-fluoro-5-(2-fluoro-4-iodophenylamino)-8-methylpyrido[2,3-d]pyrimidine-4,7(3H,8H)-dione (Example 18) as an off-white solid. The recovered feedstock was reacted under the same reaction conditions to give a second batch of product. The total yield was 347 mg (33%).1H NMR (400 MHz, DMSO-J6) δ ppm 3.36-3.40 (m, 1H), 3.43-3.50 (m, 1H), 3.58 (s, 3H), 3.61-3.72 (m, 1H), 3.72-3.82 (m, 1H), 4.27-4.37 (m, 1H) , 4.78-4.87 (m, 1H), 5.14 (d, J = 5.81 Hz, 1H), 6.93-7.03 (m, 1H), 7.53 (d, J = 8.84 Hz, 1H), 7.65-7.74 (m, 1H), 8.52 (s, 1H), 10.25 (d, J = 10.1 Hz, 1H). The calculated value for [M + H] C17H15F2IN4O4 is 505; the measured value is 505.
![(R)-3-(2,3-DIHYDROXYPROPYL)-5-(2-FLUORO-4-IODOPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180601/GIF/1035555-51-1.gif)
1035555-51-1
4 suppliers
inquiry

1035555-63-5
96 suppliers
$26.00/500μg
Yield: 33%
Reaction Conditions:
with Selectfluor in N,N-dimethyl-formamide;acetonitrile at 20; for 0.166667 h;
Steps:
18
Example 18: (i?)-3-(2,3-Dihydroxypropyl)-6-fluoro-5-(2-fluoro-4-iodophenylamino)-8- methylpyrido[2,3-d]pyrimidine-4,7(3/f,8H)-dione; To a solution of (i?)-3-(2,3-dihydroxypropyl)-5-(2-fluoro-4-iodophenylamino)- 8-methylpyrido[2,3-d]pyrimidine-4,7(3/f,8H)-dione (example 6) (1 g, 2.06 mmol, 1 eq) in DMF (19 mL) was added dropwise a mixture of Selectfluor (801 mg, 2.26 mmol, 1.1 eq)
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED WO2008/79814, 2008, A2 Location in patent:Page/Page column 122-123
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1035555-63-5
96 suppliers
$26.00/500μg
1122-67-4
24 suppliers
inquiry

1035555-63-5
96 suppliers
$26.00/500μg
![3-Benzyl-5-hydroxy-8-methyl-3H,8H-pyrido[2,3-d]pyrimidine-4,7-dione](/CAS/20180601/GIF/1035556-23-0.gif)
1035556-23-0
6 suppliers
inquiry

1035555-63-5
96 suppliers
$26.00/500μg
![3-BENZYL-5-(2-FLUORO-4-NITROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-25-2.gif)
1035556-25-2
4 suppliers
inquiry

1035555-63-5
96 suppliers
$26.00/500μg