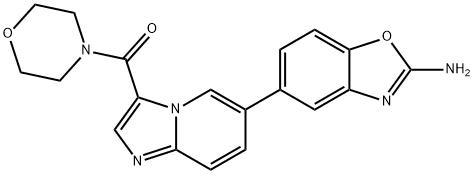
INK1117 synthesis
- Product Name:INK1117
- CAS Number:1268454-23-4
- Molecular formula:C19H17N5O3
- Molecular Weight:363.37
![(2-Aminobenzo[d]oxazol-5-yl)-boronic acid hydrochloride](/CAS/GIF/1404480-15-4.gif)
1404480-15-4
![Methanone, (6-?bromoimidazo[1,?2-?a]?pyridin-?3-?yl)?-?4-?morpholinyl-](/CAS/20200611/GIF/1434089-03-8.gif)
1434089-03-8

1268454-23-4
1. assemble a 5L three-necked, round-bottomed flask equipped with a mechanical stirrer, thermocouple probe, nitrogen/vacuum inlet, and reflux condenser, and place in a heating jacket. 2. add 1,4-dioxane (3.75 L) and water (1.25 L) to the flask at room temperature. 3. (2-Amino-1,3-benzoxazol-5-yl)boronic acid hydrochloride (250 g) and (6-bromo-5H-imidazo[2,1-b][1,3]oxazin-3-yl)(morpholinyl)methanone (210 g) were added sequentially at room temperature. 4. The reaction mixture was stirred at room temperature for 10 minutes. 5. Sodium carbonate (300 g) was added followed by Pd(PPh3)4 (47 g) at room temperature. 6. the reactor was sprayed with nitrogen for about 30 minutes under stirring to deoxygenate the reaction mixture by 5 to 6 vacuum/nitrogen cycles. 7. The reaction mixture was heated to reflux (88 °C to 102 °C) and stirred for 5 to 8 hours under light nitrogen bubbling. 8. The progress of the reaction was monitored by HPLC. 9. Upon completion of the reaction, the reaction mixture was cooled to 75-80°C. 10. 10. Water (3.75 L) and ethyl acetate (1.25 L) were added at 75-80 °C. 11. The mixture was cooled to room temperature and stirred for 2 hours. 12. The mixture was filtered and the collected solid was washed sequentially with water (2 x 1.25 L), methanol (1.25 L) and ethyl acetate (2 x 1.25 L). 13. Suspend the solid in ethyl acetate (1.5L) for 1 hour at room temperature. 14. Filtered the suspension and washed the solid with ethyl acetate (250mL). 15. Repeat steps 13 and 14 twice. 16. The solid obtained was dried under vacuum to obtain the crude product (250 g, 85% yield; HPLC purity 98.1%; Pd content 1831 ppm). 17. Purification of crude product: the crude product (250 g) was suspended in methanol (2.5 L) and HCl (158 mL) at room temperature. 18. The mixture was heated to 40 to 45°C to form a slightly turbid solution. 19. Charcoal (250 g) was added and stirred at 40 to 45°C for 30 minutes. 20. The hot solution was filtered through a polymer pad fitted with a 2-inch bed of diatomaceous earth, and the filter cake was washed with methanol (3 x 500 mL). 21. The filtrate was refilled into a flask (Pd content: 330 ppm). 22. the solution was heated to 40 to 45 °C and charcoal (50 g) and silica thiols (50 g) were added. 23. After stirring at 40 to 45°C for 30 minutes, the hot solution was filtered through a polymer pad and the filter cake was washed with methanol (250 mL). 24. Steps 22 and 23 were repeated once. 25. Concentrate under vacuum at 30 to 35°C to about 2.5L. 26. The solution was cooled to 10 to 15 °C and the pH was adjusted to 8-9 by adding concentrated ammonia solution (200 mL). 27. cool the reaction mixture to 0 to 5°C and stir for 1 to 2 hours. 28. The mixture was filtered and the filter cake was washed sequentially with water (2 x 500 mL) and methanol (2 x 500 mL). 29. Transfer the solid to a round bottom flask and suspend in ethyl acetate (2.5 L) for 2 to 3 hours at room temperature. 30. The solid was collected by filtration and washed with ethyl acetate (750mL). 31. The solid was dried under vacuum at about 50 °C to constant weight to afford the target compound (180 g, 61% yield; HPLC purity 98.5%; 1H NMR (DMSO-d6, 300 MHz) δ 9.1 (s, 1H), 8.1 (s, 1H), 7.8-7.65 (m, 2H), 7.60-7.40 (m, 4H), 7.3- 7.2 (m, 1H), 3.8-3.6 (m, 8H); 1 ppm Pd.
![(2-Aminobenzo[d]oxazol-5-yl)-boronic acid hydrochloride](/CAS/GIF/1404480-15-4.gif)
1404480-15-4
19 suppliers
inquiry
![Methanone, (6-?bromoimidazo[1,?2-?a]?pyridin-?3-?yl)?-?4-?morpholinyl-](/CAS/20200611/GIF/1434089-03-8.gif)
1434089-03-8
2 suppliers
inquiry

1268454-23-4
86 suppliers
$25.00/1mg
Yield: 85%
Reaction Conditions:
Stage #1:2-amino-benzooxazole-5-boronic acid hydrochloride;C12H12BrN3O2 in 1,4-dioxane;water at 20; for 0.166667 h;
Stage #2: with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in 1,4-dioxane;water at 20 - 102;Inert atmosphere;Reflux;
Steps:
6 Preparation of (6-(2-aminobenzo [d] oxazol-5 -yl)imidazo [ 1 ,2-a]pyridin-3 - yl)(morpholino)methanone (Formula I)
[00342] Example6[00343] Formula I[00344] Preparation of (6-(2-aminobenzo [d] oxazol-5 -yl)imidazo [ 1 ,2-a]pyridin-3 - yl)(morpholino)methanone (Formula I):[00345] 1. A 5 L three-neck round bottom flask was equipped with a mechanical stirrer, thermocouple probe, Nitrogen / vacuum inlet and reflux condenser and placed into a heating mantle.[00346] 2. 1,4-Dioxane (3.75 L) and water (1.25 L) were added at room temperature.[00347] 3. Compound 9 (250 g) and Compound 3 (210 g) were added to the flask at room temperature.[00348] 4. The reaction mixture was stirred for 10 min at room temperature.[00349] 5. Sodium carbonate (300 g) was added to the flask followed byPd(PPh3)4 (47 g) undemitrogen at room temperature.[00350] 6. With stirring, the reactor was sparged with nitrogen for -30 minutes at [00351] 7. The reaction mixture was deoxygenated by performing 5 to 6 vacuum / nitrogen cycles.[00352] 8. The reaction mixture was heated to reflux (88°C to 102°C) under slight nitrogen bubbling and stirredfor 5 to 8 hours.[00353] 9. The reaction was monitored by HPLC.[00354] 10. Upon completion, the reaction mixture was cooled to 75 - 80°C.[00355] 11. Water (3.75 L) and ethyl acetate (1.25 L) were added to the reaction mixture at 75 - 80°C.[00356] 12. The resulting mixture was cooled to room temperature and stirred for 2 hours at room temperature.[00357] 13. The mixture was filtered and the solid collected was washed with water (2x 1.25 L), methanol (1.25 L) andethyl acetate (2 x 1.25 L).[00358] 14. The solidwas suspended in ethyl acetate (1.5 L) at room temperature for 1 hour.[00359] 15. The suspension was filtered and the solidcollected was washed with ethyl acetate (250 mL).[00360] 16. This process was repeated two more times.[00361] 17. The solidobtained was dried under vacuum to give a crude product (250 g, 85% yield; HPLC purity 98.1%; Pd level 1831 ppm).[00362] Further purification of the crude product:[00363] 18. The crude product (250g) was suspended in methanol (2.5 L) and HCl (158 mL) at room temperature.[00364] 19. The mixture was heated to 40 to 45°C to give a slightly cloudy solution.[00365] 20. Charcoal (250 g) was added to the reaction mixture at 40 to 45°C. [00366] 21. The resulting mixture was stirred for 30 minutes at 40 to 45 °C.[00367] 22. The hot solution was filtered through a poly pad with 2 inch celite bed and the cake was washed withmethanol (3 x 500 mL).[00368] 23. The solution was recharged to the flask (Pd level: 330 ppm).[00369] 24. The solution was heated to 40 to 45°C.[00370] 25. Charcoal (50 g) and silica thiol (50 g) were added at 40 to 45°C.[00371] 26. The mixture was stirred for 30 minutes at 40 to 45°C.[00372] 27. The hot solution was filtered through a poly pad and the cake was washed with methanol (250 mL)[00373] 28. This process was repeated one more time.[00374] 29. The methanol was removed under vacuum at 30 - 35°C to ~2.5 L.[00375] 30. The solution was cooled to 10 to 15°C.[00376] 31. Concentrated aqueous ammonia solution (200 mL) was added until reaching pH 8-9.[00377] 32. The reaction mixture was cooled to 0 to 5°C and stirred for 1 to 2 hours at 0 to 5°C.[00378] 33. The mixture was filtered and the cake was washed with water (2 x 500 mL) and methanol (2 x 500mL).[00379] 34. The solid collected was transfered back into a round bottom flask and suspended in ethyl acetate (2.5 L) at roomtemperature for 2 to 3 hours.[00380] 35. The solid was collected by filtration and washed with ethyl acetate (750 mL).36. The solid was dried under vacuum at ~50°C to constant weight to give thecompound of Formila I as an off-white solid (180 g, 61% yield; HPLC purity 98.5%; 'HNMR (DMSO-d6, 300 MHz) ? 9.1 (s, 1H), 8.1 (s, 1H), 7.8-7.65 (2H), 7.60-7.40 (m, 4H), 7.3-7.2 (1H), 3.8-3.6 (m, 8H); Pd level lppm).
References:
INTELLIKINE, LLC;MARTIN, Michael;WORRALL, Christopher, Peter;GANCEDO, Susanna, Del Rio;REN, Pingda WO2013/71272, 2013, A1 Location in patent:Paragraph 00342-00380
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
109 suppliers
$87.00/5g

1268454-23-4
86 suppliers
$25.00/1mg
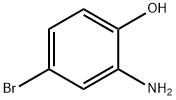
40925-68-6
258 suppliers
$6.00/1g

1268454-23-4
86 suppliers
$25.00/1mg
![5-BROMOBENZO[D]OXAZOL-2-AMINE](/CAS/GIF/64037-07-6.gif)
64037-07-6
114 suppliers
$7.00/1g

1268454-23-4
86 suppliers
$25.00/1mg
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
113 suppliers
$14.00/250mg

1268454-23-4
86 suppliers
$25.00/1mg