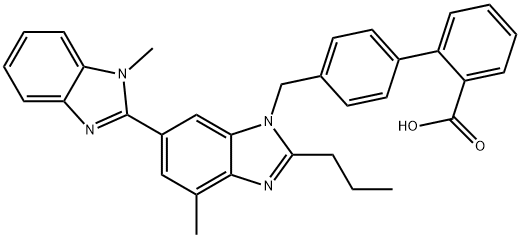
Telmisartan synthesis
- Product Name:Telmisartan
- CAS Number:144701-48-4
- Molecular formula:C33H30N4O2
- Molecular Weight:514.62
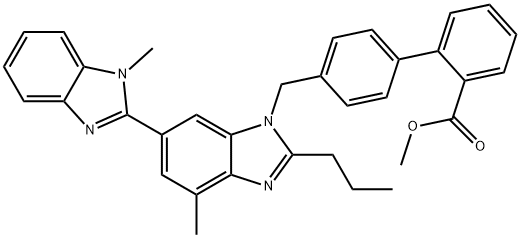
528560-93-2
126 suppliers
$75.00/25mg

144701-48-4
765 suppliers
$5.00/100mg
Yield:144701-48-4 98.6%
Reaction Conditions:
Stage #1: 4'-[[4-methyl-6-(1-methyl-1H-benzimidazol-2-yl)-2-propyl-1H-benzimidazol-1-yl]methyl]biphenyl-2-carboxylic acid methyl esterwith methanol;sodium hydroxide;water for 2 h;Heating / reflux;
Stage #2: with acetic acid in water;
Steps:
1
In a 1000 ml three-necked round bottom flask equipped with a reflux condenser, a thermometer and a magnetic stirrer, 4-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid methyl ester (50 g, 0.095 mole) was charged, and methanol (300 ml) was added followed by addition of water (26 ml) and 47% NaOH solution (27 ml). The mixture was refluxed for 2 hours. Part of the solvent was evaporated and water (500 ml) was added in portions at 85° C. to afford a solution. The insoluble matter was removed by filtration and the mixture was neutralized with a solution of acetic acid (31.7 ml) in water (75 ml). The thus obtained crude Telmisartan cake was collected by filtration and washed with water to obtain 150 g of wet Telmisartan, which was dried under vacuum to obtain 48 g of the crude product in 98.6% yield, having a purity of 97%.
References:
US2006/276525,2006,A1 Location in patent:Page/Page column 7
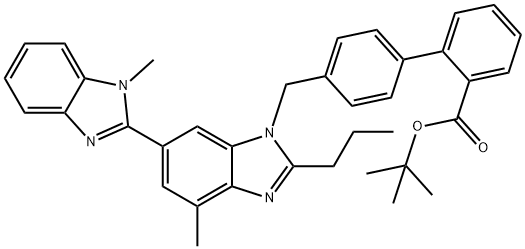
144702-26-1
99 suppliers
$82.00/50mg

144701-48-4
765 suppliers
$5.00/100mg
![[1,1'-Biphenyl]-2-carboxylic acid, 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-, hydrochloride (1:1)](/CAS/20211123/GIF/515815-48-2.gif)
515815-48-2
1 suppliers
inquiry

144701-48-4
765 suppliers
$5.00/100mg
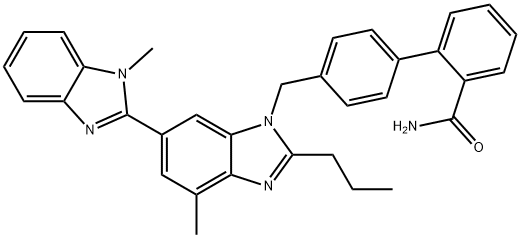
915124-86-6
74 suppliers
$185.00/100mg

144701-48-4
765 suppliers
$5.00/100mg
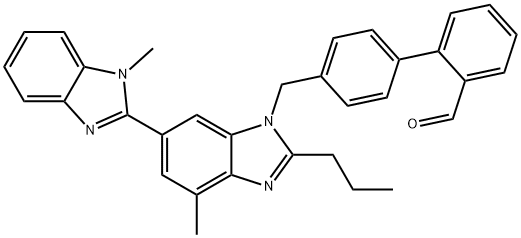
172525-90-5
1 suppliers
inquiry

144701-48-4
765 suppliers
$5.00/100mg