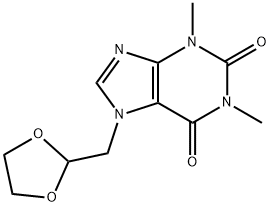
Doxofylline synthesis
- Product Name:Doxofylline
- CAS Number:69975-86-6
- Molecular formula:C11H14N4O4
- Molecular Weight:266.25
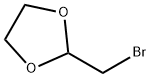
4360-63-8
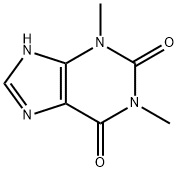
58-55-9

69975-86-6
The general procedure for the synthesis of 7-((1,3-dioxolan-2-yl)methyl)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione from 2-bromomethyl-1,3-dioxolane and 1,3-dimethyl-1H-purine-2,6(3H,9H)-dione was carried out as follows: to a reactor, 13 kg (72.16 mol) of 1,3- dimethyl-1H-purine-2,6(3H,9H)-dione (theophylline), 8 L of acetone, 5.77 kg (144.32 mol) of sodium hydroxide and 0.7 kg (2.16 mol) of tetrabutyl ammonium bromide. After stirring for 10 min, 14.47 kg (86.64 mmol) of 2-bromomethyl-1,3-dioxolane (bromoacetaldehyde acetal) was added. The mixture was refluxed for 6.3 h and the progress of the reaction was monitored by thin-layer chromatography (unfolding reagent ratio acetone: dichloromethane = 3:1). Upon completion of the reaction, the solvent was removed by distillation under reduced pressure, washed three times with saturated sodium chloride solution, and the residue was recrystallized from anhydrous ethanol to give 17.28 kg (64.98 mol) of 7-((1,3-dioxolan-2-yl)methyl)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione (doxophyllotoxin) in 90% yield and purity over 98.5%.
Yield:69975-86-6 90%
Reaction Conditions:
with tetrabutylammomium bromide;sodium hydroxide in acetone for 6.3 h;Reflux;Large scale;Reagent/catalyst;Solvent;
Steps:
1.c c. Preparation of doxofylline
To the reaction tank, 13 Kg (72.16 mol) of theophylline, 8L of acetone, 5.77 Kg of sodium hydroxide (144.32 mol) and 0.7 Kg of tetrabutylammonium bromide (2.16 mol) were added to the reaction vessel. After stirring for 10 min, 14.47 Kg of bromoacetaldehyde ethylene acetal (86.64mmol) was added. The mixture was refluxed for 6.3h and the end point of the reaction was monitored by thin layer chromatography (acetone: dichloromethane = 3: 1). After the completion of the reaction, the solvent was distilled off under reduced pressure, washed three times with saturated brine, and the residue was recrystallized from absolute ethanol to give 17.28 kg of doxofylline (64.98 mol) in a yield of 90%, the content is above 98.5%.
References:
Beijing Yifang Biological Technology Co., Ltd.;Wang, Jiao;Shi, Qingdong CN105418612, 2016, A Location in patent:Paragraph 0029; 0041; 0042
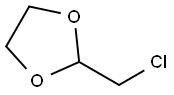
2568-30-1
170 suppliers
$24.00/25g

58-55-9
612 suppliers
$5.00/1g

69975-86-6
457 suppliers
$25.00/1g
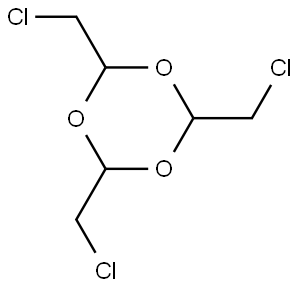
1129-52-8
6 suppliers
inquiry

58-55-9
612 suppliers
$5.00/1g
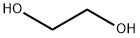
107-21-1
1374 suppliers
$10.00/25g

69975-86-6
457 suppliers
$25.00/1g