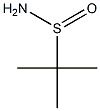
tert-Butanesulfinamide synthesis
- Product Name:tert-Butanesulfinamide
- CAS Number:146374-27-8
- Molecular formula:C4H11NOS
- Molecular Weight:121.2
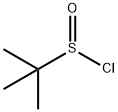
31562-43-3
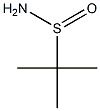
146374-27-8
1. A solution of 2-methylpropanethiol (14.1 cm3, 125 mmol) in DCM (50 mL) was cooled at -40°C. The solution was then purified and the solution was allowed to cool for 30 minutes. At an interval of 30 min, a suspension (50 mL) of m-chlorobenzoic acid (63.2 g, 256 mmol, 2.05 eq.) was added. Under vigorous stirring (exothermic reaction), a solution of DCM (500 mL) pre-cooled to -78 °C was added in batches. The resulting white suspension was stirred at -30°C overnight, then cooled to -78°C and filtered. The filtrate was concentrated under reduced pressure and dried under high vacuum to give crude tert-butyl sulfinic acid (13.5 g, 88%). 2. Thionyl chloride (40.3 mL, 552.5 mmol, 5 eq.) was added to the above sulfinic acid at -40 °C under nitrogen protection. The yellow solution was slowly warmed to room temperature and stirred for 2 hours. Concentration under high vacuum gave tert-butyl sulfinyl chloride (12.9 g, 83%) as a brown oil. 3. 3. DCM solution of tert-butylsulfinyl chloride (582 mL) was slowly added to ammonia (38% aqueous, 582 mL, 220 mmol, 2.4 eq.) and stirred for 1 hour at room temperature. The aqueous layer was washed with saturated NaCl solution and extracted with DCM (4 x 600 cm3). The organic phases were combined, dried over MgSO? and concentrated under reduced pressure to give tert-butylsulfinamide (9.2 g, 83%) as a white solid which could be recrystallized from hexane. Product characterization: Rf = 0.23 (EtOAc); melting point 103-104 °C [(S)-enantiomer 106 °C (TCI)]; IR (νmax/cm?1) 3231, 2799, 2959, 2928, 2869, 1677, 1570, 1475, 1461, 1364, 1309, 1193, 1132, 1030, 893; 1H NMR (300 MHz, CDCl?) δ 1.19 [9H, s, C(CH?)? , 3.96 (2H, br s, NH?); 13C NMR (75 MHz, CDCl?) δ 22.1, 55.2; MS (CI+) m/z 139 (M?+1, 800%), 122 (M?+1, 100%).
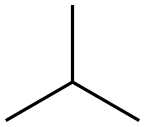
75-28-5
132 suppliers
$40.00/1g
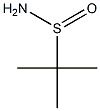
146374-27-8
241 suppliers
$9.00/5g
Yield:-
Reaction Conditions:
with amidosulfurous acid at 180 - 220; under 1520.1 - 2280.15 Torr;
Steps:
At a temperature of 180-220 ° C under a pressure of 2-3 atmospheres,The raw material sulfinic acid amine and isobutane through the demethylation of tert-butyl sulfinate amines. The purity of the crude product to meet the general reaction needs, can be directly into the reaction.
References:
CN105753754,2016,A Location in patent:Paragraph 0006

31562-43-3
110 suppliers
$45.00/50mg
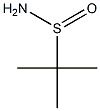
146374-27-8
241 suppliers
$9.00/5g
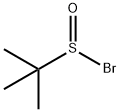
31562-44-4
0 suppliers
inquiry
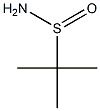
146374-27-8
241 suppliers
$9.00/5g
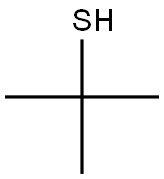
75-66-1
148 suppliers
$18.00/25mL
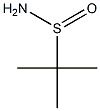
146374-27-8
241 suppliers
$9.00/5g
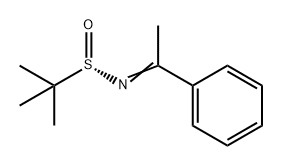
842121-02-2
0 suppliers
inquiry
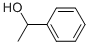
13323-81-4
102 suppliers
inquiry
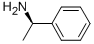
3886-69-9
587 suppliers
$14.00/25G
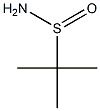
146374-27-8
241 suppliers
$9.00/5g
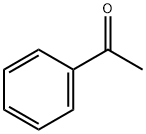
98-86-2
795 suppliers
$9.00/5g