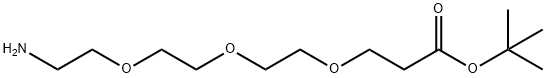
TERT-BUTYL 12-AMINO-4 7 10-TRIOXA-DODECA synthesis
- Product Name:TERT-BUTYL 12-AMINO-4 7 10-TRIOXA-DODECA
- CAS Number:252881-74-6
- Molecular formula:C13H27NO5
- Molecular Weight:277.36
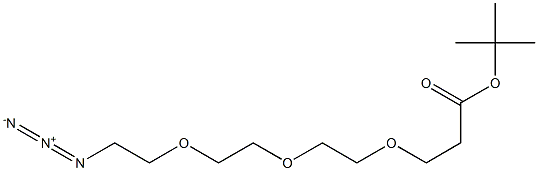
252881-73-5

252881-74-6
The general procedure for the synthesis of tert-butyl 12-amino-4,7,10-trioxa-dodecanoate from tert-butyl 3-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)propionate was as follows: Raney-Ni (7.5 g, suspended in water) was washed sequentially with water (three times) and isopropanol (three times), and was subsequently mixed with tert-butyl 3-(2-(2-(2- azidoethoxy)ethoxy ) ethoxy)propionic acid tert-butyl ester (5.0 g, 16.5 mmol) in isopropanol. The reaction mixture was stirred at room temperature under hydrogen atmosphere for 16 hours. After completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad and the pad was washed with isopropanol. The filtrate was concentrated and purified by column chromatography (eluent: 5-25% methanol/dichloromethane) to afford the light yellow oily product tert-butyl 12-amino-4,7,10-trioxa-dodecanoate (2.60 g, 57% yield). Mass spectrometry (ESI) m/z calculated value C13H28NO5 [M+H]+ 279.19; measured value: 279.19.

252881-73-5
86 suppliers
inquiry

252881-74-6
141 suppliers
$21.00/100mg
Yield:252881-74-6 95%
Reaction Conditions:
with hydrogen;sodium hydroxide in water;isopropyl alcohol at 20; pH=8; for 15 h;Inert atmosphere;
References:
Tavernaro, Isabella;Hartmann, Sebastian;Sommer, Laura;Hausmann, Heike;Rohner, Christian;Ruehl, Martin;Hoffmann-Roeder, Anja;Schlecht, Sabine [Organic and Biomolecular Chemistry,2015,vol. 13,# 1,p. 81 - 97]
![Propanoic acid, 3-[2-[2-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]ethoxy]ethoxy]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1234367-88-4.gif)
1234367-88-4
1 suppliers
inquiry

252881-74-6
141 suppliers
$21.00/100mg
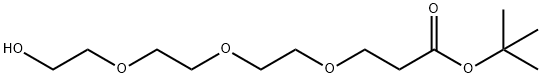
186020-66-6
157 suppliers
$25.00/1g

252881-74-6
141 suppliers
$21.00/100mg
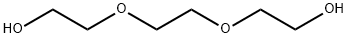
112-27-6
782 suppliers
$6.00/100g

252881-74-6
141 suppliers
$21.00/100mg
1663-39-4
260 suppliers
$10.00/5g

252881-74-6
141 suppliers
$21.00/100mg