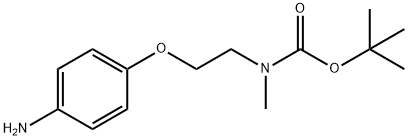
tert-butyl 2-(4-aminophenoxy)ethylmethylcarbamate synthesis
- Product Name:tert-butyl 2-(4-aminophenoxy)ethylmethylcarbamate
- CAS Number:1170071-27-8
- Molecular formula:C14H22N2O3
- Molecular Weight:266.34
![Carbamic acid, N-methyl-N-[2-(4-nitrophenoxy)ethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1170071-26-7.gif)
1170071-26-7
0 suppliers
inquiry

1170071-27-8
4 suppliers
inquiry
Yield:1170071-27-8 95%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol at 50; for 1 h;
Steps:
82
Example 82Synthesis of te/*-butyl 2-(4-isocyanatophenoxy)ethyl(methyl)carbamate[00236] To a mixture 2-(methylamino) ethanol (5.0 g, 66.5 mmol) in 15ml of ethyl acetate was added a solution of (BoC)2O (14.5 g, 66.5 mmol) in 5 mi of ethyl acetate dropwise with cooling in an ice bath The resulting mixture was stirred at room temperature for 2 hours, and the solvent was removed by evaporation under reduced pressure. The residue was dissolved in ethyl acetate, washed with water, dried over Na^SO4 and filtered. After removing the solvent, the crude tert-butyl 2-hydroxyethyl(methyl)carbamate was used without further purification for the next reaction (10.5 g, 90%) A solution of diisopropyl azodicarboxylate (5.22 g, 25.9 mmol) in 5 ml of THF was added dropwise to a solution of 4-nitryl phenol (3.0 g, 21 56 mmol), tert-butyl 2-hydroxyethyl(methyl)carbamate (4.53 g,
References:
WO2009/85562,2009,A1 Location in patent:Page/Page column 99-100

24424-99-5
869 suppliers
$13.50/25G

1170071-27-8
4 suppliers
inquiry
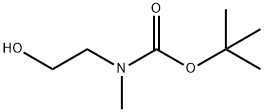
57561-39-4
165 suppliers
$14.00/1g

1170071-27-8
4 suppliers
inquiry