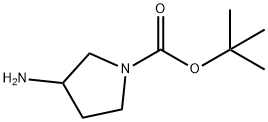
tert-Butyl 3-aminopyrrolidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-aminopyrrolidine-1-carboxylate
- CAS Number:186550-13-0
- Molecular formula:C9H18N2O2
- Molecular Weight:186.25
862906-31-8

186550-13-0
Step (ii): preparation of tert-butyl 3-aminopyrrolidine-1-carboxylate (Formula 49) To a stirred solution of tert-butyl 3-hydroxypyrrolidine-1-carboxylate (Formula 48, 1.15 g, 4.16 mmol) in methanol (16.6 mL) was added 10% palladium/carbon catalyst (345 mg). The reaction mixture was stirred for 16 hours under a hydrogen atmosphere (hydrogen pressure applied via a double-layer balloon). Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad to remove the catalyst and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel fast column chromatography to afford the target compound tert-butyl 3-aminopyrrolidine-1-carboxylate (Formula 49, 640 mg) in 82% yield. 1H-NMR (CDCl3): δ 3.65-3.45 (m, 3H), 3.45-3.33 (m, 1H), 3.20-3.0 (m, 1H), 2.12-2.02 (m, 1H), 1.80-1.65 (m, 1H), 1.46 (s, 9H). Mass spectrum (m/z): 187 [M+H]+.

24424-99-5
869 suppliers
$13.50/25G
79286-79-6
124 suppliers
$20.00/250mg

186550-13-0
231 suppliers
$8.00/1g
Yield:186550-13-0 98%
Reaction Conditions:
in chloroform at 20; for 1 h;
Steps:
A9 Example A9; 3RS- [4-AMINO-5- (2, 6-DIFLUORO-BENZOYL)-THIAZOL-2-YLAMINO]-PYRROLIDINE-1-CARBOXYLIC acid TERF-BUTYL ESTER
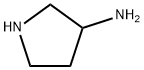
The starting materials were prepared as follows : 3RS-AMINO-PYRROLIDINE-1-CARBOXYLIC ACID TEFF-BUTYL ESTER To a solution of 3-aminopyrrolidine (0. 86 g, 10 MMOL) in CHCI3 (50 mi) at 0 C was added dropwise a solution of di-t-butyl dicarbonate ((BOC) 2O ; 2. 06 g, 10 MMOL) in CHCI3 (50 ML). The mixture stirred at room temperature for 1 hour, and then washed with brine, dried over K2CO3, filtered, and concentrated to give 1. 8 g of yellow oil in 98% yield, which was used without further purification. 'H NMR : 8 3. 60-3. 28 (m, 4H), 3. 02 (m, 1 H), 2. 04 (m, 1 H), 1. 64 (m, 1 H), 1. 45 (s, 9H), 1. 45-1. 20 (m, 2H)
References:
PFIZER INC. WO2004/74283, 2004, A1 Location in patent:Page 38

862906-31-8
2 suppliers
inquiry

186550-13-0
231 suppliers
$8.00/1g
113451-52-8
10 suppliers
inquiry

186550-13-0
231 suppliers
$8.00/1g
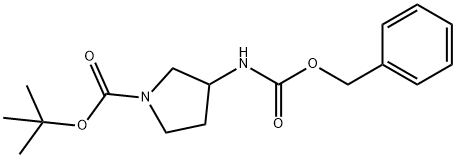
325775-36-8
27 suppliers
$300.00/250mg

186550-13-0
231 suppliers
$8.00/1g
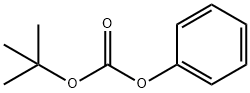
6627-89-0
144 suppliers
$8.00/5g

79286-79-6
124 suppliers
$20.00/250mg

186550-13-0
231 suppliers
$8.00/1g