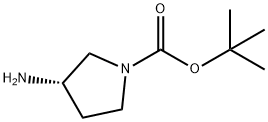
(S)-(-)-1-tert-Butoxycarbonyl-3-aminopyrrolidine synthesis
- Product Name:(S)-(-)-1-tert-Butoxycarbonyl-3-aminopyrrolidine
- CAS Number:147081-44-5
- Molecular formula:C9H18N2O2
- Molecular Weight:186.25
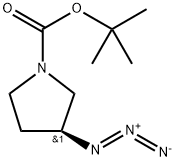
125552-56-9

147081-44-5
To a solution of compound 178 (2.0 g, 9.42 mmol, 1.0 eq.) in methanol (40 mL) was added palladium carbon (1.0 g, 0.94 mmol, 0.1 eq.). The reaction mixture was subjected to hydrogenation at atmospheric pressure and room temperature for 6 hours. After completion of the reaction, the reaction mixture was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure. The target compound (S)-1-Boc-3-aminopyrrolidine was obtained as a yellow oil (1.7 g, 97% yield).

125552-56-9
8 suppliers
inquiry

147081-44-5
340 suppliers
$14.00/1g
Yield:147081-44-5 97%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol at 20; under 760.051 Torr; for 6 h;
Steps:
73.B
To a solution of product 178 (2.0 g, 9.42 mmol, 1.0 eq.) in 40 mL of methanol, palladium on charcoal (1.0 g, 0.94 mmol, 0.1 eq.) is added. The reaction medium is submitted to hydrogenation at atmospheric pressure and at room temperature for 6 hours. The reaction medium is then filtered on celite and concentrated under dry conditions under reduced pressure. The expected compound is obtained as a yellow oil (1.7 g, 97%).
References:
Vetoquinol SA US2009/221565, 2009, A1 Location in patent:Page/Page column 51
202267-26-3
3 suppliers
inquiry

147081-44-5
340 suppliers
$14.00/1g
127423-61-4
64 suppliers
$15.00/100mg

147081-44-5
340 suppliers
$14.00/1g

24424-99-5
869 suppliers
$13.50/25G

147081-44-5
340 suppliers
$14.00/1g
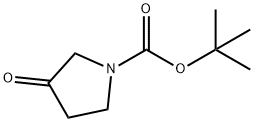
101385-93-7
484 suppliers
$5.00/1g

147081-44-5
340 suppliers
$14.00/1g