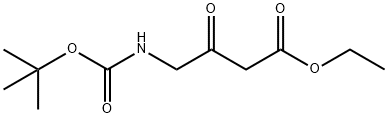
TERT-BUTYL 3-(ETHOXYCARBONYL)-2-OXOPROPYLCARBAMATE synthesis
- Product Name:TERT-BUTYL 3-(ETHOXYCARBONYL)-2-OXOPROPYLCARBAMATE
- CAS Number:67706-68-7
- Molecular formula:C11H19NO5
- Molecular Weight:245.27
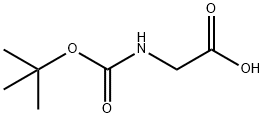
4530-20-5
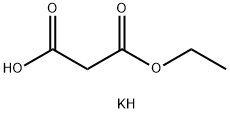
6148-64-7

67706-68-7
(1) Carbonyldiimidazole (10.2 g, 63.1 mmol) was added to a suspension of 2-(tert-butoxycarbonylamino)acetic acid (10 g, 57.1 mmol) in tetrahydrofuran (100 mL) and the reaction was stirred at room temperature for 2 hours. Subsequently, magnesium chloride (5.4 g, 57.1 mmol) and monoethyl malonate potassium salt (9.7 g, 57.1 mmol) were sequentially added to the reaction system and stirring was continued for 1 h at 60 °C. The reaction was completed by filtration. After completion of the reaction, the insoluble material was removed by filtration, and the filtrate was diluted with ethyl acetate (200 mL), washed with 1N hydrochloric acid, saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride in turn, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography [eluent: n-hexane-ethyl acetate (2:1)] to afford ethyl 4-(tert-butoxycarbonylamino)-3-oxobutanoate (11.9 g, 48.5 mmol, 85% yield) as a colorless oil.1H-NMR (CDCl3) δ: 1.29 (3H, t, J = 7.4 Hz), 1.45 (2H, s) , 3.87 (2H, s), 3.96 (3H, s), 4.21 (2H, q, J = 7.2 Hz), 7.08 (1H, d, J = 8.7 Hz), 7.39 (9H, s), 3.49 (2H, s), 4.14 (2H, d, J = 5.8 Hz), 4.21 (2H, q, J = 7.4 Hz), 5.15-5.24 (1H, br).

4530-20-5
572 suppliers
$5.00/5 g

67706-68-7
36 suppliers
$62.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: triethylamine / tetrahydrofuran / 0.5 h / -30 - -20 °C
2: 1.) diisopropylamine, n-BuLi / 1.) THF, -70 deg C, 30 min, 2.) THF, -65 deg C, 20 min
References:
Kim, Hwa-Ok;Olsen, Richard K.;Choi, Ok-Soon [Journal of Organic Chemistry,1987,vol. 52,# 20,p. 4531 - 4536]

4530-20-5
572 suppliers
$5.00/5 g

6148-64-7
411 suppliers
$5.00/10g

67706-68-7
36 suppliers
$62.00/250mg

24424-99-5
867 suppliers
$13.50/25G

86578-55-4
0 suppliers
inquiry

67706-68-7
36 suppliers
$62.00/250mg
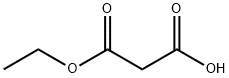
1071-46-1
293 suppliers
$5.00/1g
![Carbamic acid, N-[2-(1H-imidazol-1-yl)-2-oxoethyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/18185-77-8.gif)
18185-77-8
1 suppliers
inquiry

67706-68-7
36 suppliers
$62.00/250mg

6148-64-7
411 suppliers
$5.00/10g
![Carbamic acid, N-[2-(1H-imidazol-1-yl)-2-oxoethyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/18185-77-8.gif)
18185-77-8
1 suppliers
inquiry

67706-68-7
36 suppliers
$62.00/250mg